A kind of pyridine heterocyclic compound and its application as cxcr4 inhibitor
A technology of a heterocyclic compound and a pyridine ring, used in diabetic retinopathy, for, a composition comprising the compound, a heterocyclic compound, brain inflammation, related diseases, and the field of age-related macular degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Heterocyclic compounds C1 and C2, which are synthesized by the following method:
[0098]
[0099] 1) Synthesis of intermediate C1-2:
[0100] Boc-D-proline (5g, 23mmol), isopropylidene malonate (3.4g, 23mmol) and DMAP (5.7g, 46mmol) were dissolved in 70mL of dichloromethane, under stirring in an ice bath, DCC (4.8 g, 23mmol), stirred at room temperature for 48h. After filtration, 200 mL of dichloromethane and 100 mL of 1N hydrochloric acid solution were added to the filtrate for layering, and the aqueous phase was extracted with dichloromethane. The dichloromethane phases were combined, dried and spin-dried to obtain a yellow oil (9 g, crude product).
[0101] 2) Synthesis of intermediate C1-3:
[0102] Intermediate C1-2 (9 g, crude product) was dissolved in 100 mL of absolute ethanol, and stirred at reflux for 2 h. Ethanol was spin-dried to obtain a liquid, which was purified by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain a yellow oil...
Embodiment 2
[0111] According to the synthesis method of Example 1, Boc-D-proline was replaced by Boc-L-proline in step 1, and heterocyclic compounds C3 and C4 were synthesized.
Embodiment 3
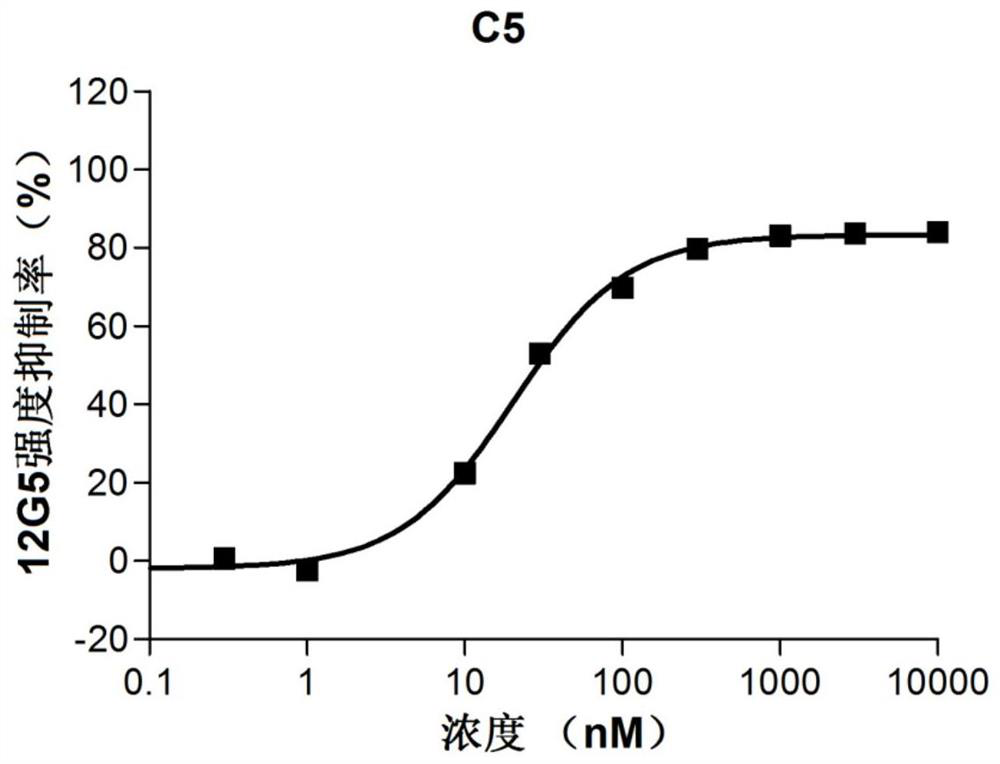
[0113] Heterocyclic compound C5, which is synthesized by the following method:
[0114]
[0115] 1) Synthesis of intermediate C5-1:
[0116] Intermediate C1-3 (1g, 3.5mmol) and acetamidine hydrochloride (990mg, 10.5mmol) were dissolved in 200mL of ethanol, sodium ethoxide (480mg, 7mmol) was added, and stirred under reflux overnight. Acetic acid was added to adjust the pH to acidic, and extracted three times with dichloromethane (100 mL). The organic phase was dried and spin-dried to obtain a liquid, which was purified by column chromatography (dichloromethane:methanol=20:1) to obtain a colorless oily liquid (930mg, 95%)
[0117] 2) Synthesis of intermediate C5-2:
[0118] Intermediate C5-2 (920mg, 3.3mmol), triethylamine (3.3g, 33mmol), N-methylpiperazine (500mg, 5mmol) were dissolved in 20mL acetonitrile, PyBOP (1.9g, 3.4mmol) was added, and stirred at reflux Overnight, 200 mL of dichloromethane and 200 mL of aqueous sodium bicarbonate were added to separate layers, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com