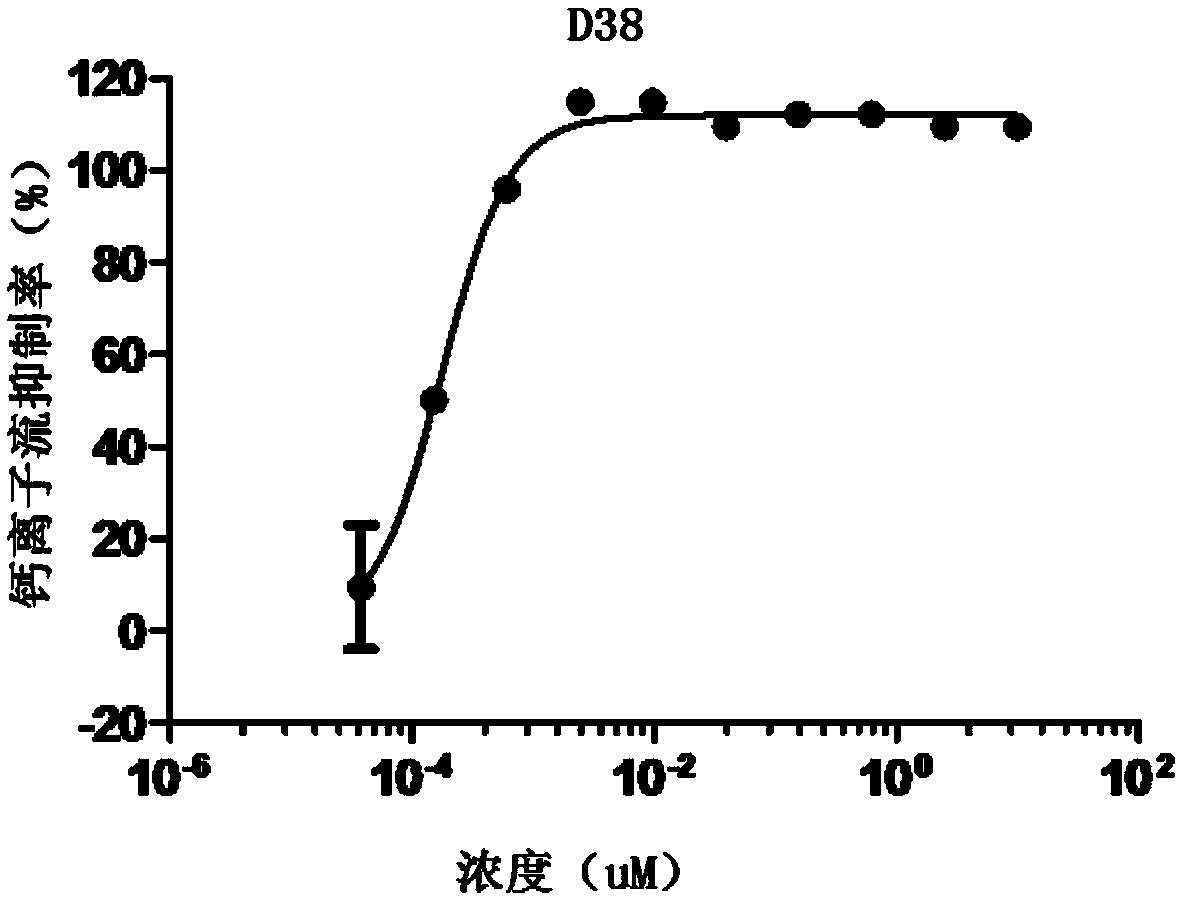
A class of heterocyclic compounds with CXCR4 signal channel inhibition activity, and applications thereof
A heterocyclic compound, a technology for inhibiting activity, applied to the composition containing the compound, related to diseases, used in the fields of heterocyclic compounds, age-related macular degeneration, brain inflammation, and diabetic retinopathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
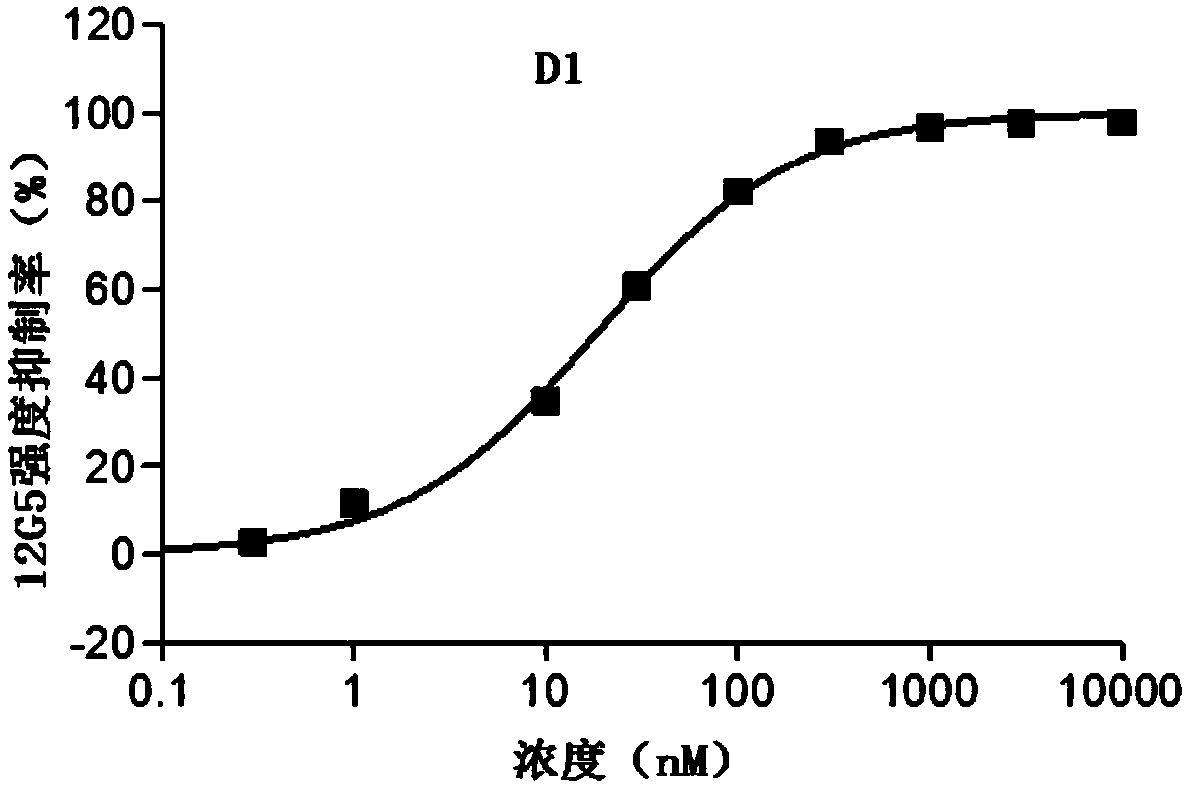
[0095] Heterocyclic compound D1, which is synthesized by the following method:
[0096]
[0097] 1) Synthesis of intermediate D1-1
[0098] Ethyl chloroacetoacetate (16.5 g, 100 mmol) and acetamidine hydrochloride (10 g, 106 mmol) were successively dissolved in ethanol (150 mL). 1,8-Diazabicycloundec-7-ene (30.4 g, 200 mmol) was slowly added in an ice-water bath, and stirred overnight at room temperature. Drain the solvent, add dichloromethane (120mL) to dilute, wash with saturated brine (30mL*3), dry the organic phase, and drain the solvent. The residue was dissolved in phosphorus oxychloride (20 mL), and stirred under reflux at 110° C. for 30 min. After cooling to room temperature, most of the phosphorus oxychloride was removed, and the residue was dissolved in ethyl acetate (20 mL) and added dropwise to ice water (100 mL). The aqueous phase was extracted with ethyl acetate (30 mL*3), and the organic phases were combined and dried. After the solvent was spin-dried, th...
Embodiment 2
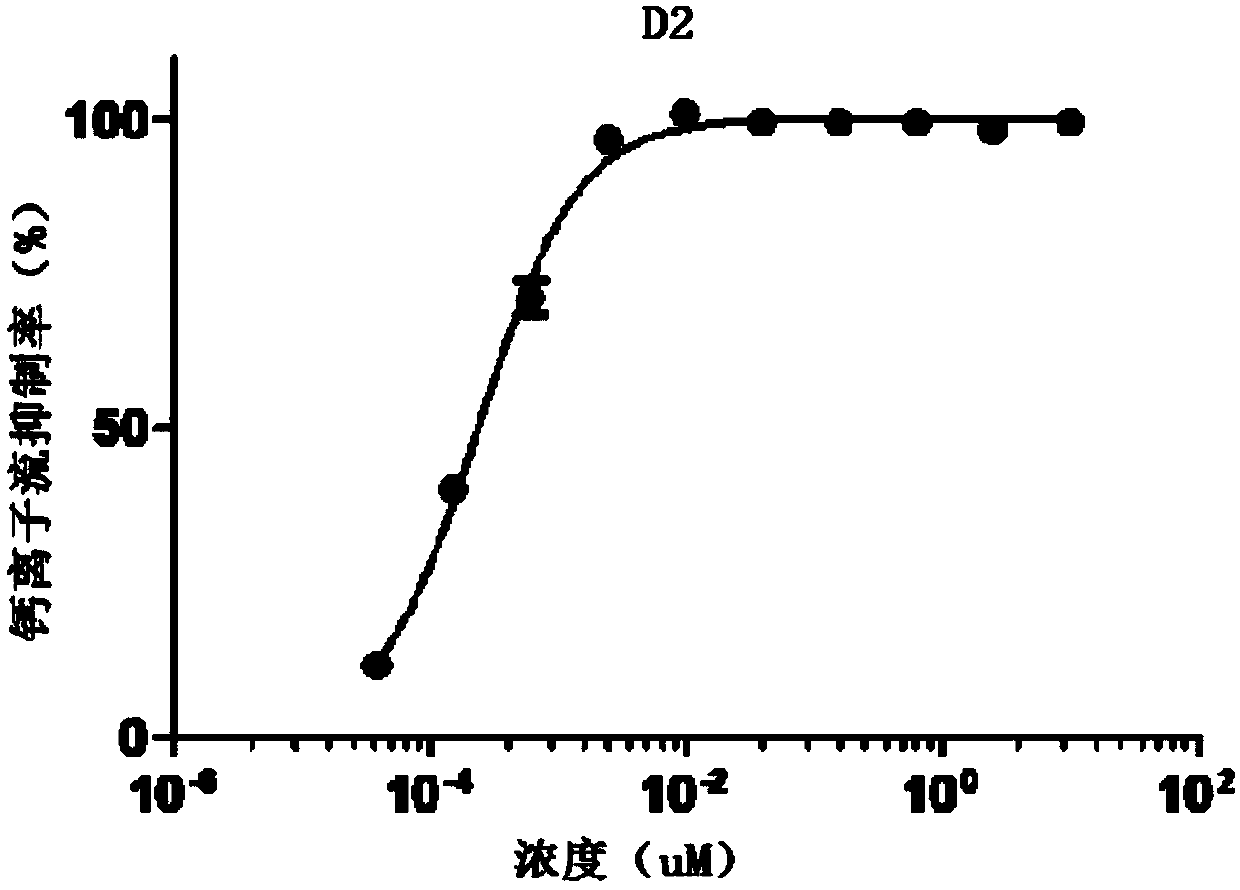
[0106] Heterocyclic compound D2, which is synthesized by the following method:
[0107]
[0108] 1) Synthesis of intermediate D2-1
[0109] 8-aminoquinoline (200mg, 1.4mmol), intermediate D1-1 (273mg, 1.5mmol), potassium iodide (23mg, 0.14mmol), N,N-diisopropylethylamine (452mg, 3.5mmol) were added into acetonitrile (5 mL) and react overnight at room temperature. Potassium carbonate (386mg, 2.8mmol) was added, the temperature was raised to 90°C, and the reaction was carried out overnight. The reaction solution was spin-dried, added with saturated sodium bicarbonate solution (50 mL), and extracted with dichloromethane (3*20 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried. The residue was purified by column chromatography (dichloromethane:methanol:ammonia water=100:2:1) to obtain a yellow oily intermediate (230 mg, 58%). 1 H NMR (400MHz, CDCl 3 )δ8.79(d, J=2.8Hz, 1H), 8.11(d, J=8.0Hz, 1H), 7.44(dd, J=8...
Embodiment 3
[0115] Heterocyclic compound D3, which is synthesized by the following method:
[0116]
[0117] 1) Synthesis of intermediate D3-1
[0118] The intermediate 8-aminoquinoline (2.9g, 20mmol) was added to absolute ethanol, heated to 90°C, slowly added dropwise into iodomethane (3.4g, 24mmol), sealed and stirred overnight. The solvent was spin-dried, and saturated sodium bicarbonate (50mL) and dichloromethane (60mL) were added, the aqueous phase was extracted twice with dichloromethane (60mL*2), the organic phases were combined, concentrated under reduced pressure, and purified by column chromatography (petroleum Ether:ethyl acetate=50:1) to obtain yellow oil D3-1 (0.90g, 29.8%).
[0119] 2) Synthesis of intermediate D3-2
[0120] Intermediate D3-1 (158mg, 1.9mmol), dissolved in 10mL acetonitrile, added potassium iodide (31.5mg, 0.19mmol), potassium carbonate (524mg, 3.8mmol), D1-1 (219mg, 2.84mmol), reacted overnight at 80°C , spin-dried to obtain an oil, which was purified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com