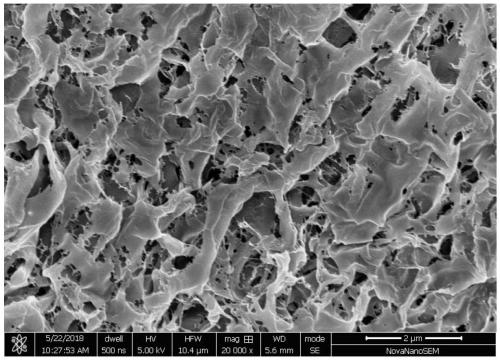
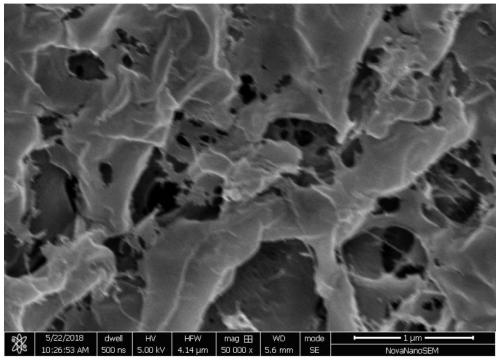
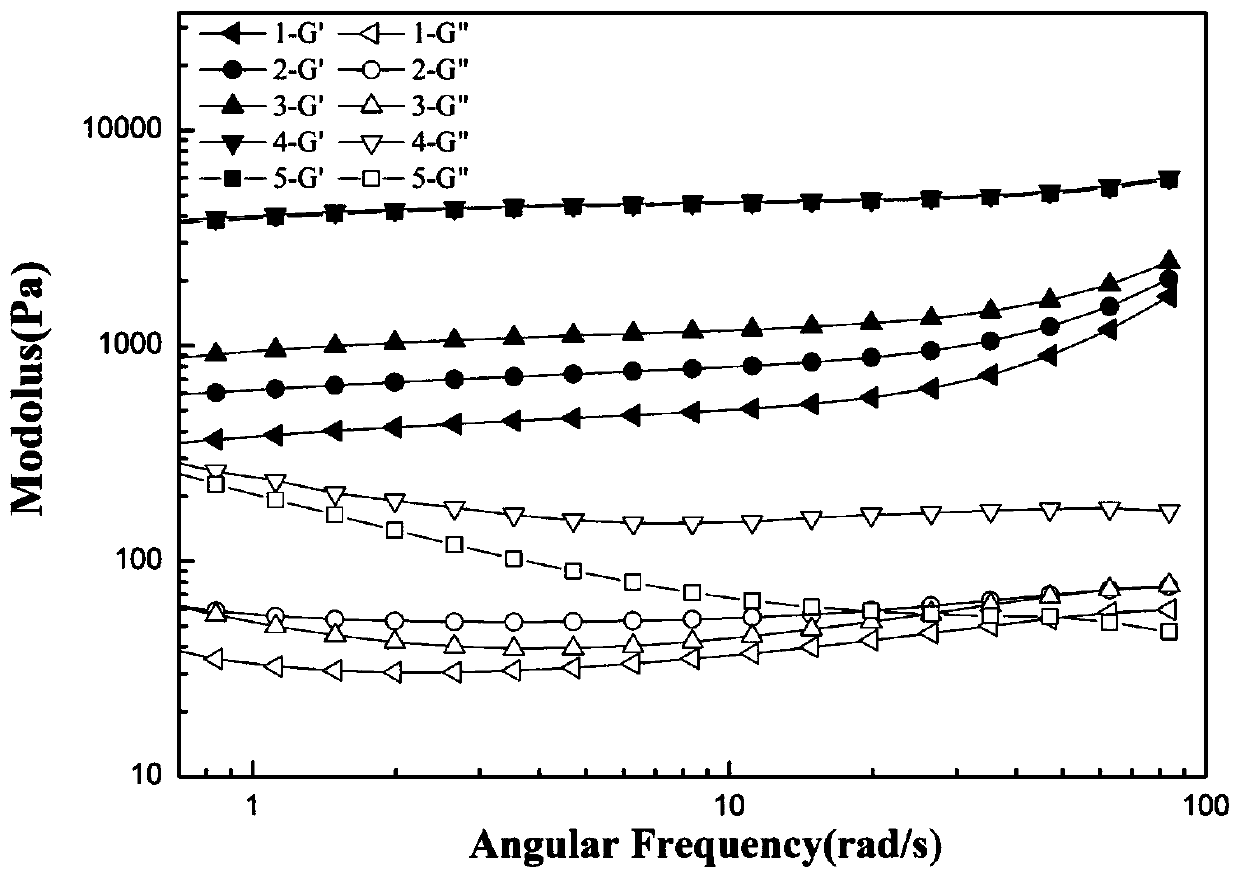
Injectable hydrogel material and preparation method and application thereof
A technology of gel material and injection water, which is applied in X-ray contrast agent preparation, pharmaceutical formulation, general/multifunctional contrast agent, etc., and can solve unfavorable post-injection gel fixed-point molding, long-term exercise tolerance, and biocompatibility Poor sex and biodegradability, slow coagulation of injectable hydrogels, etc., to achieve enhanced in vivo traceability, high integrity and adjustability, and good resistance to exercise fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Accurately weigh 10.0g chitosan and 10g sodium hydroxide in a three-necked flask, add 100mL isopropanol solvent, and swell at 50°C for 3 hours; accurately weigh 10.0g monochloroacetic acid and dissolve it in 20mL isopropanol middle. Slowly add chloroacetic acid solution dropwise into chitosan lye to react for 12 hours; pour 200mL of absolute ethanol to wash the product, filter and dry to obtain carboxymethyl chitosan;
[0040] (2) Accurately weigh 1.0 g of guar gum, dissolve it in 100 mL of water, add 10 mL of sodium periodate solution (0.1 g / mL) dropwise in the dark at room temperature, and react for 2 h. Centrifuge to remove slag, precipitate the product with ethanol, and dry to obtain bisaldehyde guar gum;
[0041] (3) Measure 10 mL of iohexol injection, add 1 mL of sodium periodate solution (0.1 g / mL) dropwise in the dark at room temperature and react for 1 hour; separate and purify by column chromatography to obtain an aldylated iohexol solution;
[0042] (4)...
Embodiment 2- Embodiment 5
[0048] The difference between Example 2-Example 5 and Example 1 is that in step (5), the formulation of component B is different, see Table 1, and the rest of the processes are identical.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com