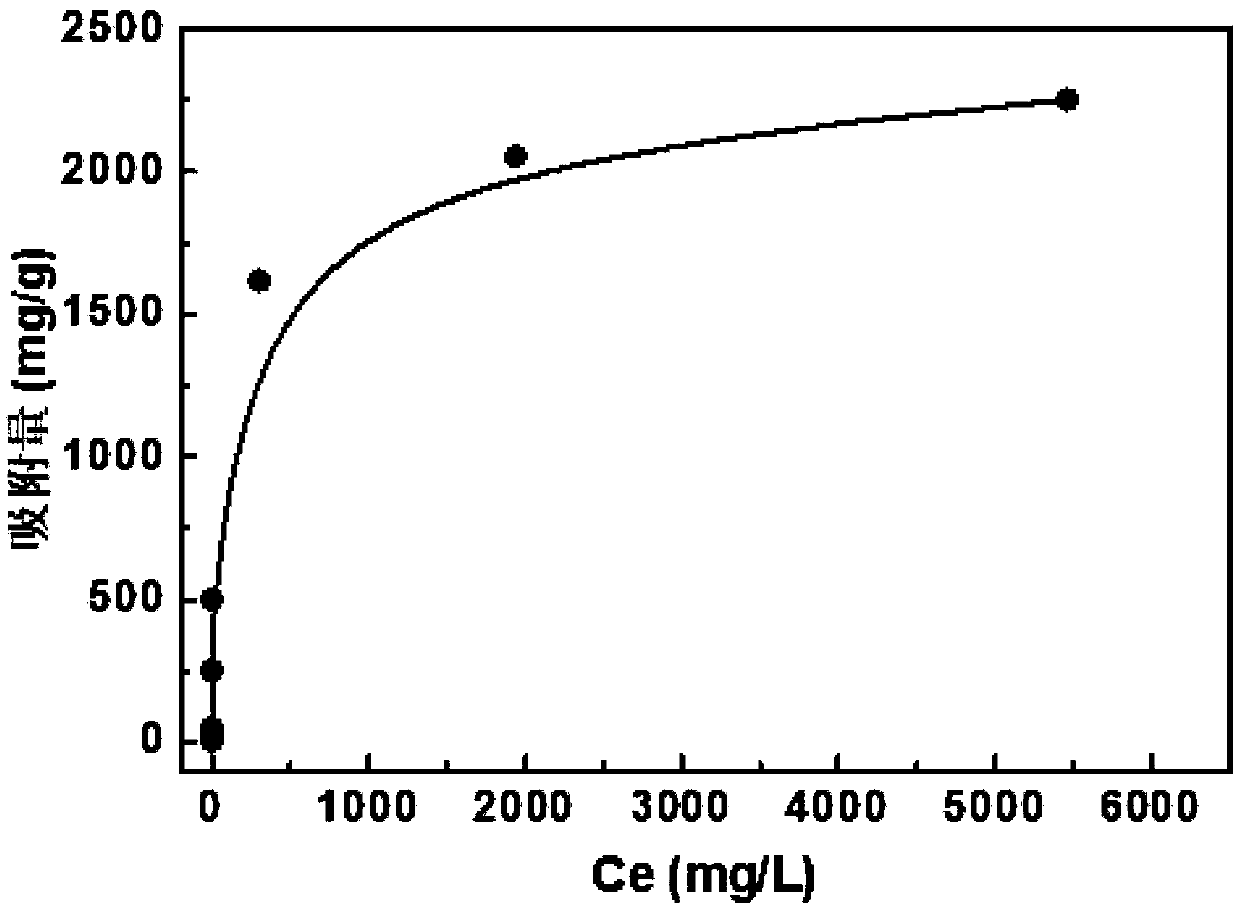
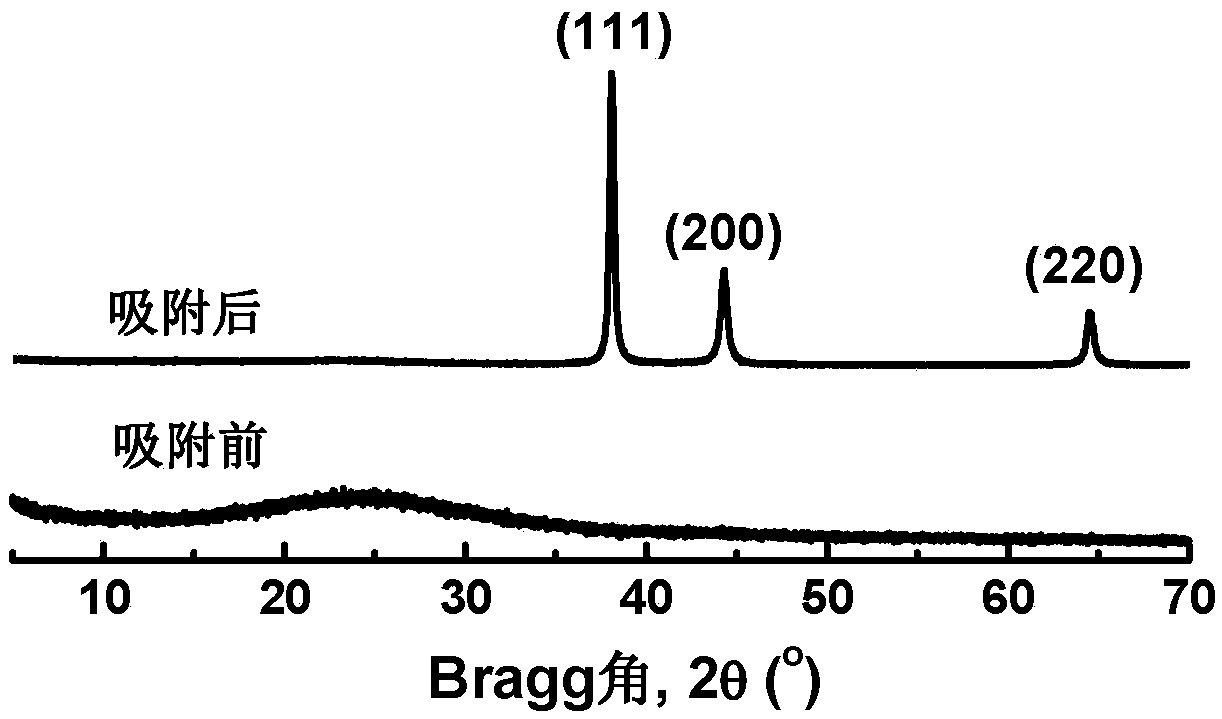
Application of polyaninophenol as precious metal adsorbent
A technology of polyaniline and precious metal catalysts, which is applied in the direction of adsorption water/sewage treatment, process efficiency improvement, water/sludge/sewage treatment, etc., to achieve high enrichment recovery efficiency and shorten the recovery path effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment a
[0065] Add 20mL of deionized water, 2.05g of m-phenylenediamine and 0.11g of hydroquinone into a 100mL beaker in sequence, place it on a magnetic stirrer and stir to dissolve it fully, and obtain a mixed monomer solution with a feeding molar ratio of 95 / 5 , and then placed in a -5°C water bath for later use. Add 150mL of deionized water and 4.76g of sodium persulfate successively into a 200mL beaker, and stir on a magnetic stirrer to fully dissolve it. The sodium persulfate solution was added dropwise to the above mixed monomer solution at a rate of 1 drop / 3 seconds. After the dropwise addition, continue to stir the reaction until 12h, centrifuge the reaction system, and use deionized water several times in turn until the supernatant liquid after centrifugation is clarified and washed with BaCl 2 The solution does not detect SO 4 2- ion so far. The product was transferred to a watch glass and dried at 60° C. to constant weight, and the yield of polyaniline was 57.7%.
Embodiment b
[0067] In a 100mL beaker, add 20mL of 1M hydrochloric acid aqueous solution, 3.39g of phenylenediamine sulfonate and 0.252g of pyroglucinol in turn, place it on a magnetic stirrer and stir to make it fully dissolve, and obtain a mixed monomer solution with a feeding molar ratio of 90 / 10 , and then placed in a 10°C water bath for later use. Add 150mL of 1M hydrochloric acid aqueous solution and 9.1g of ammonium persulfate successively into a 200mL beaker, place on a magnetic stirrer and stir to fully dissolve. The ammonium persulfate solution was added dropwise to the above mixed monomer solution at a rate of 1 drop / 3 seconds. After the dropwise addition, continue to stir the reaction until 12h, centrifuge the reaction system, and use deionized water several times in turn until the supernatant liquid after centrifugation is clarified and washed with BaCl 2 The solution does not detect SO 4 2- ion so far. The product was transferred to a watch glass and dried at 60° C. to co...
Embodiment c
[0069] In a 100mL beaker, add 20mL of 1M nitric acid aqueous solution, 1.73g of anilinesulfonic acid and 1.74g of phenolsulfonic acid successively, place it on a magnetic stirrer and stir to make it fully dissolve, and obtain a mixed monomer solution whose feeding molar ratio is 50 / 50, and then Place in a water bath at 10°C for later use. Add 150mL of deionized water and 4.56g of ammonium persulfate successively into a 200mL beaker, place on a magnetic stirrer and stir to fully dissolve. The ammonium persulfate solution was added dropwise to the above mixed monomer solution at a rate of 1 drop / 3 seconds. After the dropwise addition, continue to stir the reaction until 72h, centrifuge the reaction system, wash several times with deionized water, transfer the product to a watch glass and dry it to constant weight at 60°C, the yield of polyaniline is 26.6% .
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com