Catalyst for catalyzing synthesis of indoxacarb key intermediate and application of catalyst
A technology for catalyzing indanes and catalysts, which is applied in the directions of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc., and can solve the problems of large amount of catalyst, high production cost, low catalyst efficiency, etc. , to achieve the effect of enhancing selectivity, reducing dosage, and facilitating industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Prepare a chiral catalyst and use the catalyst to prepare indoxacarb. The specific preparation method is as follows:
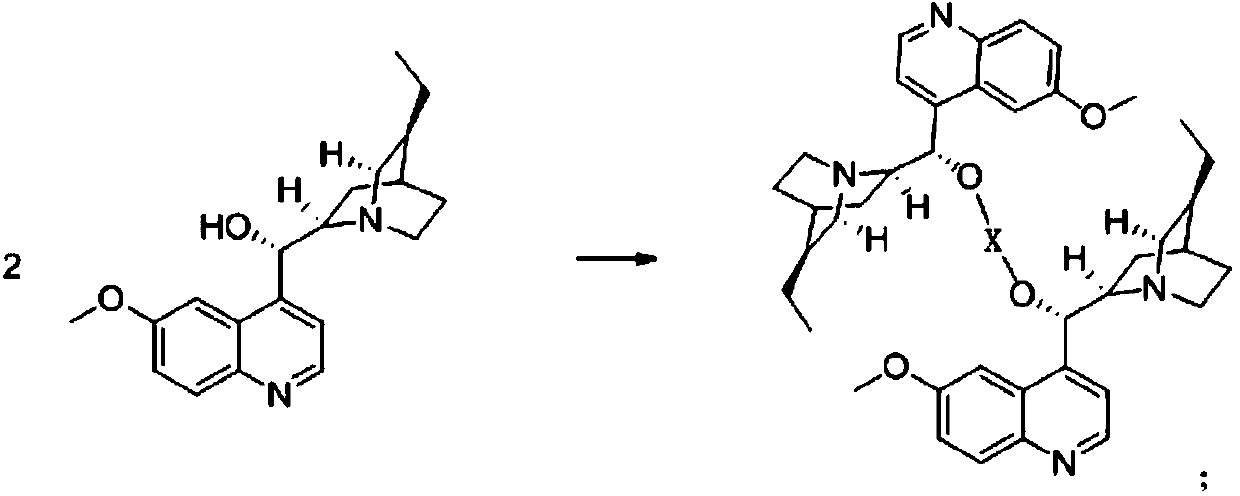
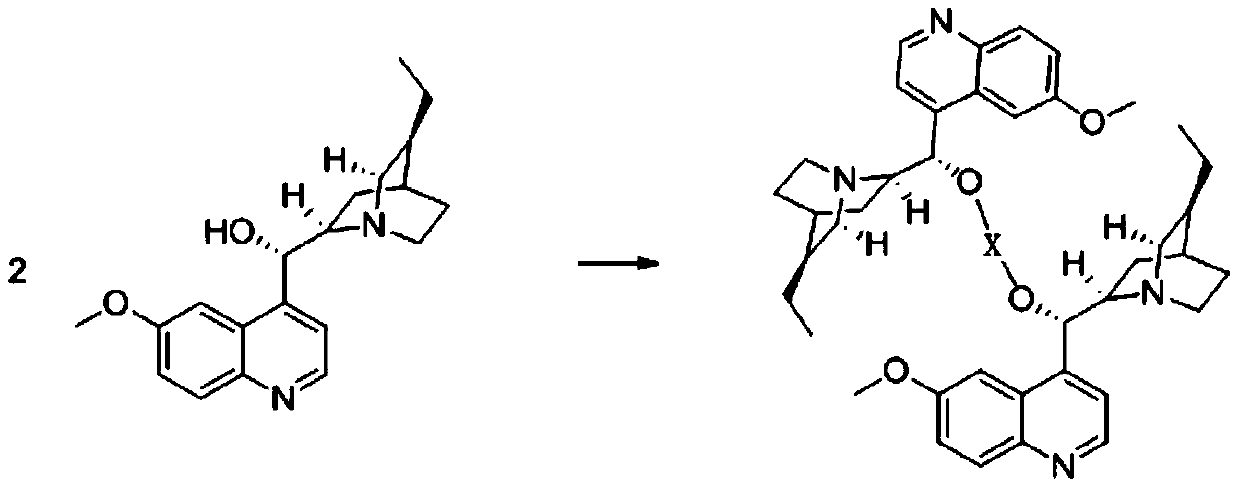
[0040] (1) Add 3.26g (0.01mol) of quinidine hydrochloride to a 1000mL clean and anhydrous reaction flask, then add 50mL of toluene, heat up to 60°C and stir to dissolve.
[0041] (2) Weigh 1.25 g (0.01 mol) of manganese chloride, and add it to the toluene solution of the above-mentioned hydroquinidine.
[0042] (3) Heating up to 80° C., reacting for 2 hours to obtain a clear and transparent chiral catalyst solution.
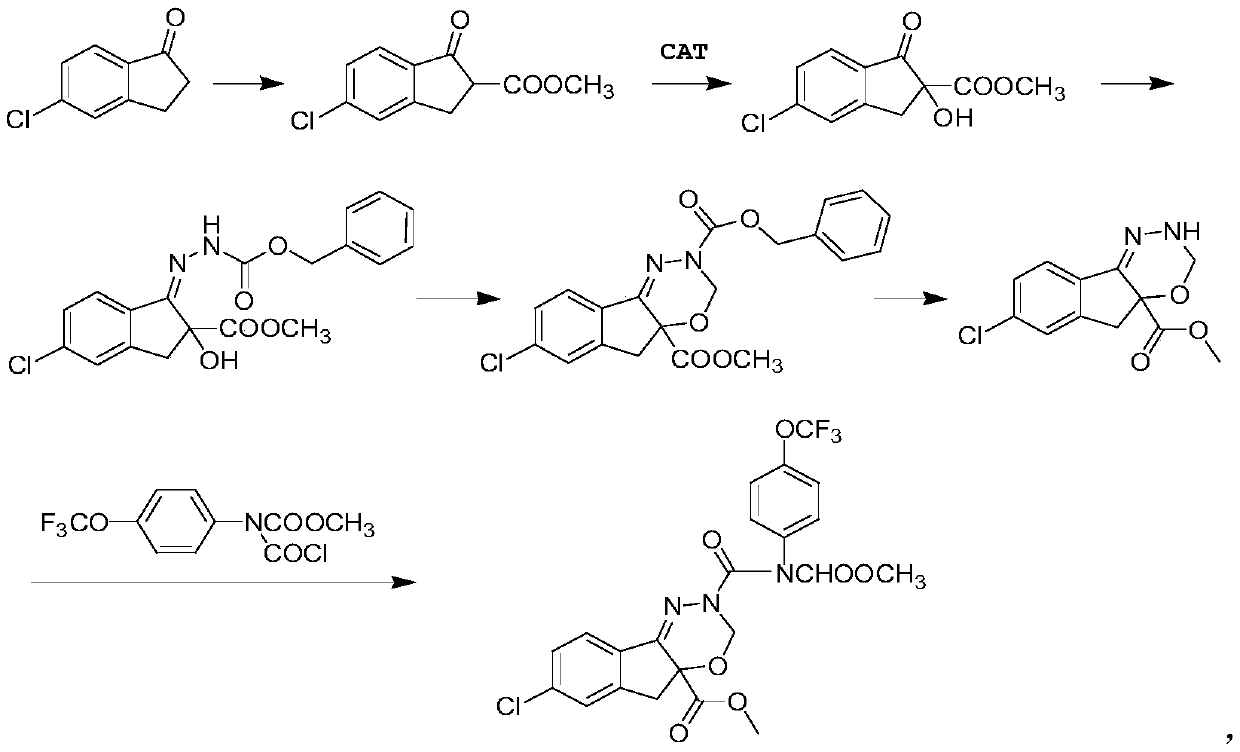
[0043] The method for preparing S-body indoxacarb using the above-mentioned catalyst can adopt the conventional synthesis route disclosed in the master’s degree thesis "Synthetic Technology and Polymorphic Form of Novel and High-efficiency Chiral Insecticide Indoxacarb" awarded by Zhejiang University in 2014. in:
[0044] (4) Add 225g tert-butyl hydroperoxide and 449g 5-chloro-2-methoxycarbonyl-1-indanone ester and 500mL toluene to the c...
Embodiment 2
[0047] Prepare a chiral catalyst and use the catalyst to prepare indoxacarb. The specific preparation method is as follows:
[0048] (1) Add 4.89g (0.015mol) of quinidine hydrochloride to a 1000mL clean and anhydrous reaction flask, then add 50mL of toluene, heat up to 60°C and stir to dissolve.
[0049] (2) Weigh 1.51 g (0.01 mol) of manganese sulfate, and add it into the above-mentioned toluene solution of quinidine hydrochloride.
[0050] (3) Heating up to 80° C., reacting for 2 hours to obtain a clear and transparent chiral catalyst solution.
[0051] The method for preparing S-body indoxacarb using the above-mentioned catalyst can adopt the conventional synthesis route disclosed in the master’s degree thesis "Synthetic Technology and Polymorphic Form of Novel and High-efficiency Chiral Insecticide Indoxacarb" awarded by Zhejiang University in 2014. in:
[0052] (4) add 113g tert-butyl hydroperoxide and 224g 5-chloro-2-methoxycarbonyl-1-indanone ester and 600mL toluene t...
Embodiment 3
[0055] Prepare a chiral catalyst and use the catalyst to prepare indoxacarb. The specific preparation method is as follows:
[0056] (1) Add 6.52g (0.02mol) of quinidine hydrochloride to a 1000mL clean and anhydrous reaction flask, then add 50mL of toluene, heat up to 60°C and stir to dissolve.
[0057] (2) Weigh 0.89g (0.01mol) of manganese hydroxide, and add it into the above-mentioned toluene solution of quinidine hydrochloride.
[0058] (3) Heating up to 80° C., reacting for 2 hours to obtain a clear and transparent chiral catalyst solution.
[0059] The method for preparing S-body indoxacarb using the above-mentioned catalyst can adopt the conventional synthesis route disclosed in the master’s degree thesis "Synthetic Technology and Polymorphic Form of Novel and High-efficiency Chiral Insecticide Indoxacarb" awarded by Zhejiang University in 2014. in:
[0060] (4) add 169g tert-butyl hydroperoxide and 336g 5-chloro-2-methoxycarbonyl-1-indanone ester and 600mL toluene to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com