Ferrous ion fluorescent probe molecule based on dansyl acid structure, preparation method and application
A fluorescent probe, ferrous ion technology, applied in the field of metal ion fluorescent probe detection, achieves the effects of high synthesis yield, wide practicability and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The method of the ferrous ion fluorescent probe molecule based on the dansulphonic acid structure, the steps are as follows:
[0039] Under ice-cooling conditions, m-chloroperoxybenzoic acid (172 mg, 1 mmol) in 10 mL of anhydrous dichloromethane was slowly added dropwise to a solution of dansyl chloride (269.5 mg, 1 mmol) in 10 mL of anhydrous dichloromethane , then raised to room temperature and reacted for 2 hours, added basic alumina (204 mg, 2 mmol) and stirred for 1 hour. The solvent was spin-dried, separated by column chromatography, the developer DCM / MeOH=4:1, and 107 mg of a yellow product was obtained, with a yield of 40%.
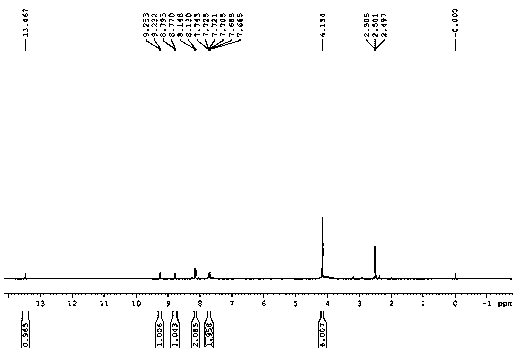
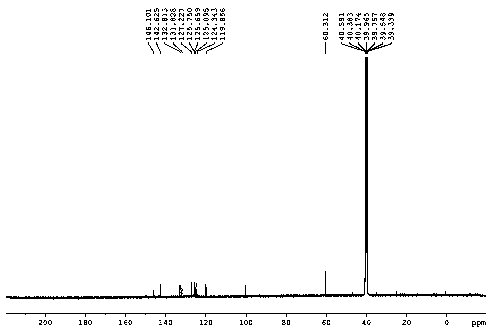
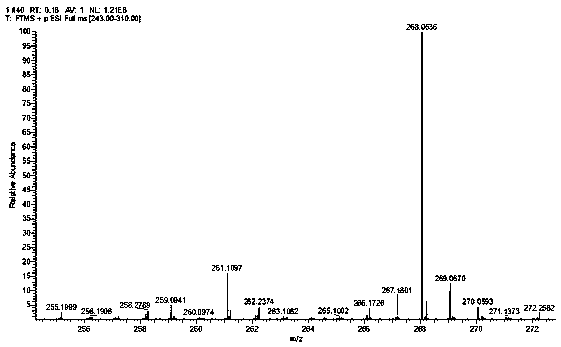
[0040] H NMR 1 H NMR (DMSO-d 6, 400 MHz) δ : 4.15 (s, 6 H), 7.72 (m, 2 H),8.14 (d, J = 7.2 Hz, 2 H), 8.78 (d, J = 9.2 Hz, 1 H), 9.24 ( d, J = 8.4 Hz, 1H), 13.47 (s, 1 H); C NMR 13 C NMR (DMSO-d 6 , 100 MHz) δ: 60.3, 119.9, 124.3, 125.1, 125.7, 125.8, 127.2, 131.8, 132.8, 142.6, 146.1; HR-ESI-MScalcd for: C 12 h 14 NO 4 S: 267.0565, ...
Embodiment 2
[0042] The method of the ferrous ion fluorescent probe molecule based on the dansulphonic acid structure, the steps are as follows:
[0043] Under ice-cooling conditions, m-chloroperoxybenzoic acid (374 mg, 2 mmol) in 15 mL of anhydrous dichloromethane was slowly added dropwise to a solution of dansyl chloride (269.5 mg, 1 mmol) in 10 mL of anhydrous dichloromethane , then raised to room temperature and reacted for 4 hours, added basic alumina (204 mg, 2 mmol) and stirred for 2 hours. The solvent was spin-dried, separated by column chromatography, and the developing solvent DCM / MeOH=5:1 to obtain 133.5 mg of a yellow product with a yield of 50%.
[0044] H NMR 1 H NMR (DMSO-d 6 , 400 MHz) δ : 4.15 (s, 6 H), 7.72 (m, 2 H),8.14 (d, J = 7.2 Hz, 2 H), 8.78 (d, J = 9.2 Hz, 1 H), 9.24 ( d, J = 8.4 Hz, 1H), 13.47 (s, 1 H); C NMR 13 C NMR (DMSO-d 6 , 100 MHz) δ: 60.3, 119.9, 124.3, 125.1, 125.7, 125.8, 127.2, 131.8, 132.8, 142.6, 146.1; HR-ESI-MScalcd for: C 12 h 14 NO 4 S: 26...
Embodiment 3
[0046] The method of the ferrous ion fluorescent probe molecule based on the dansulphonic acid structure, the steps are as follows:
[0047] Under ice-cooling conditions, m-chloroperoxybenzoic acid (430 mg, 2.5 mmol) in 15 mL of anhydrous dichloromethane was slowly added dropwise to a solution of dansyl chloride (269.5 mg, 1 mmol) in 10 mL of anhydrous dichloromethane , then raised to room temperature and reacted for 7 hours, added basic alumina (306 mg, 3 mmol) and stirred for 2 hours. The solvent was spin-dried, separated by column chromatography, the developer DCM / MeOH=6:1, and 174 mg of a yellow product was obtained with a yield of 65%.
[0048] H NMR 1 H NMR (DMSO-d 6 , 400 MHz) δ : 4.15 (s, 6 H), 7.72 (m, 2 H),8.14 (d, J = 7.2 Hz, 2 H), 8.78 (d, J = 9.2 Hz, 1 H), 9.24 ( d, J = 8.4 Hz, 1H), 13.47 (s, 1 H); C NMR 13 C NMR (DMSO-d 6 , 100 MHz) δ: 60.3, 119.9, 124.3, 125.1, 125.7, 125.8, 127.2, 131.8, 132.8, 142.6, 146.1; HR-ESI-MScalcd for: C 12 h 14 NO 4 S: 267.056...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com