A kind of pyrene 2-(2-methylthiopyridine) aniline schiff base zn 2+ Preparation and Application of Fluorescent Probes
A technology of methylthiopyridine and fluorescent probes, which is applied in the field of fluorescent probes, can solve problems such as limited applications, and achieve the effects of high sensitivity, low detection limit, and high anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1), the reaction formula of synthesizing 1-bromopyrene:
[0045]
[0046] (2), the concrete steps of synthetic 1-bromopyrene:
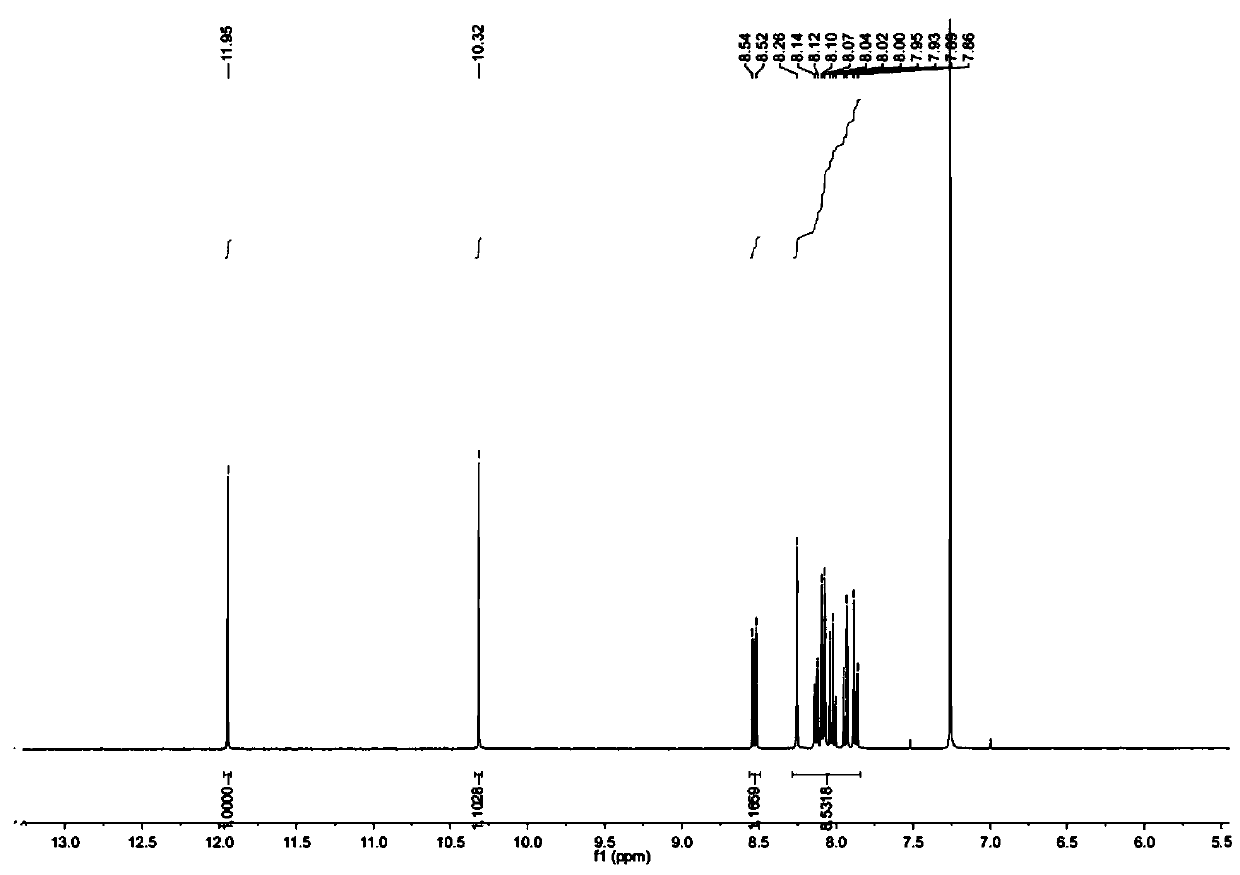
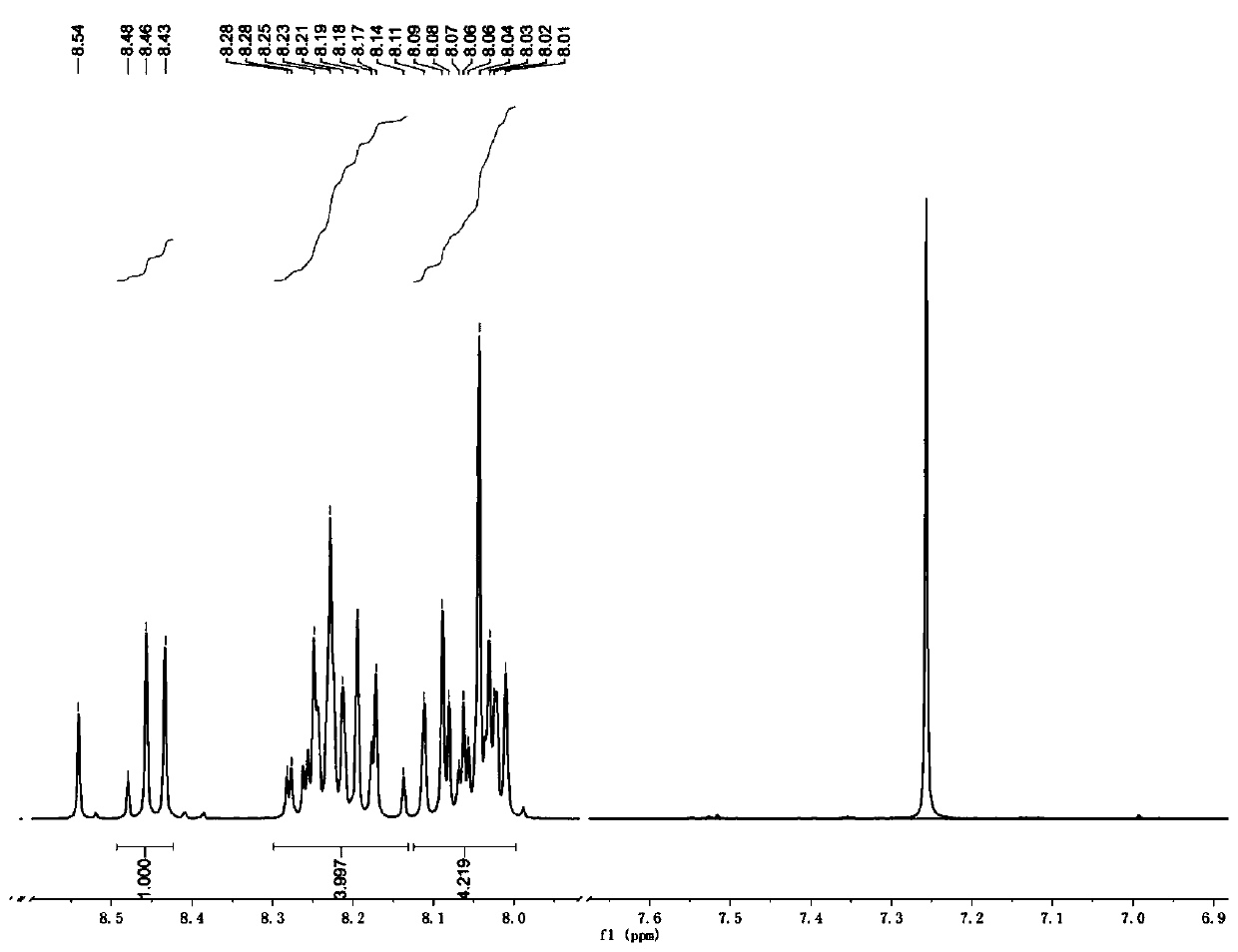
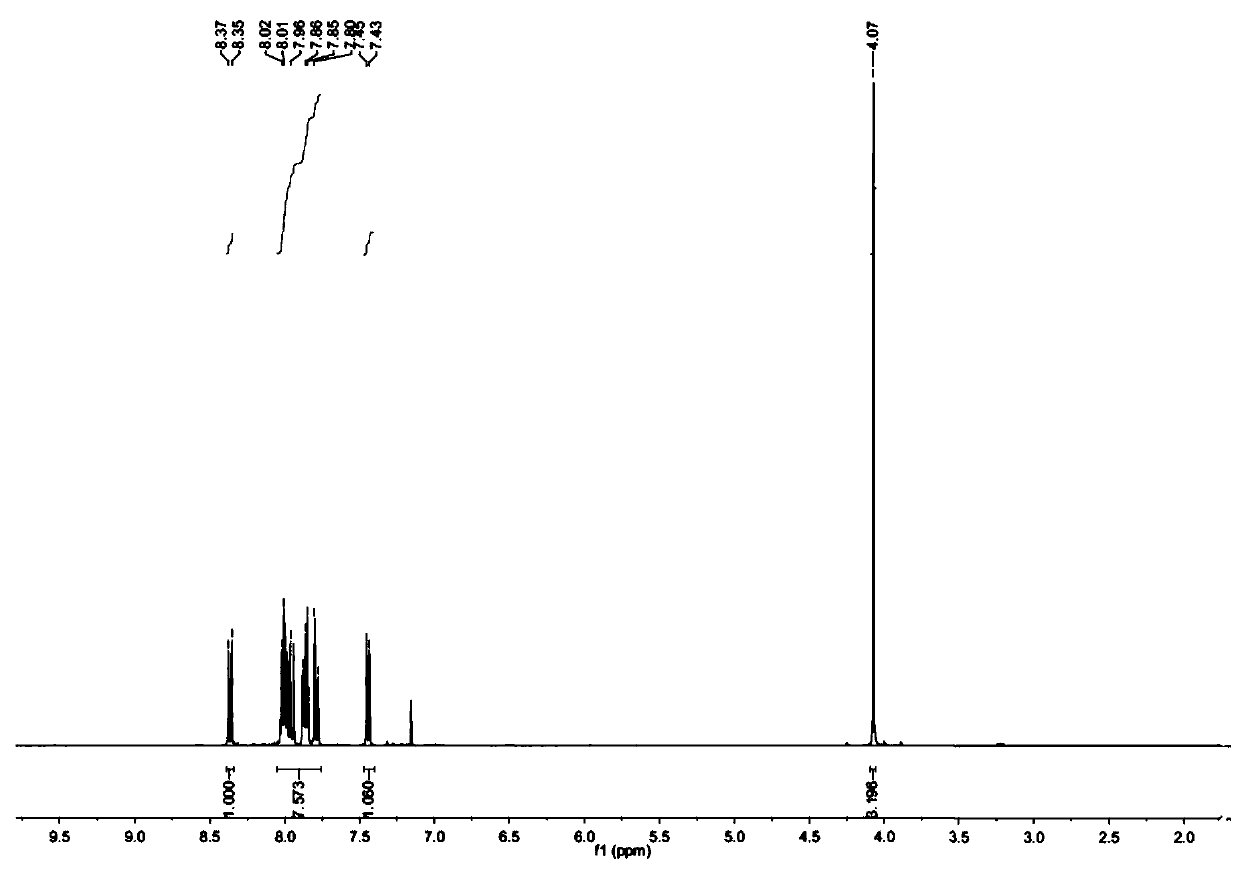
[0047] Weigh 10g pyrene and 9g NBS and dissolve in 100mL chloroform, N 2 Stir and heat to 65°C under protection, react for 10 h, TLC detects that the reaction is complete, rotary evaporation under reduced pressure, and then column chromatography with petroleum ether and ethyl acetate as eluent to obtain 1-bromopyrene. The yield is 75%. 1-Bromopyrene 1 H NMR spectrum as figure 1 shown.
[0048] (3), the reaction formula of synthesizing 1-methoxypyrene:
[0049]
[0050] (4), the concrete steps of synthetic 1-methoxypyrene:
[0051] Weigh 4g of sodium, and 100mLCH 3 OH reaction is complete, weigh 4g 1-bromopyrene and 1.2g CuI and add to the reaction system, add 30mL DMF, fill with N 2 Protected, heated to reflux to 85°C, reacted for 30h, cooled, added ice water, extracted with dichloromethane, washed with water, then dried the orga...
Embodiment 2
[0075] (1), the concrete steps of synthetic 1-bromopyrene:
[0076] Weigh 10g pyrene and 9g NBS and dissolve in 100mL chloroform, N 2 Stir and heat to 70°C under protection and react for 10 h. The reaction is complete as detected by TLC. Rotary evaporation under reduced pressure, and then column chromatography using petroleum ether and ethyl acetate as eluent to obtain 1-bromopyrene. The yield was 77%.
[0077] (2), the concrete steps of synthetic 1-methoxypyrene:
[0078] Weigh 4g of sodium, and 100mLCH 3 OH reaction is complete, weigh 4g 1-bromopyrene and 1.2g CuI and add to the reaction system, add 30mL DMF, fill with N 2 Protected, heated to reflux to 95°C, reacted for 30h, cooled, added ice water, extracted with dichloromethane, washed with water, then dried the organic phase with anhydrous sodium sulfate, filtered under reduced pressure, and the filtrate was evaporated under reduced pressure to remove the solvent, and the Ether as eluent column chromatography, in 1-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com