Synthesis and performance research of coumarins Mg2+ fluorescent probe
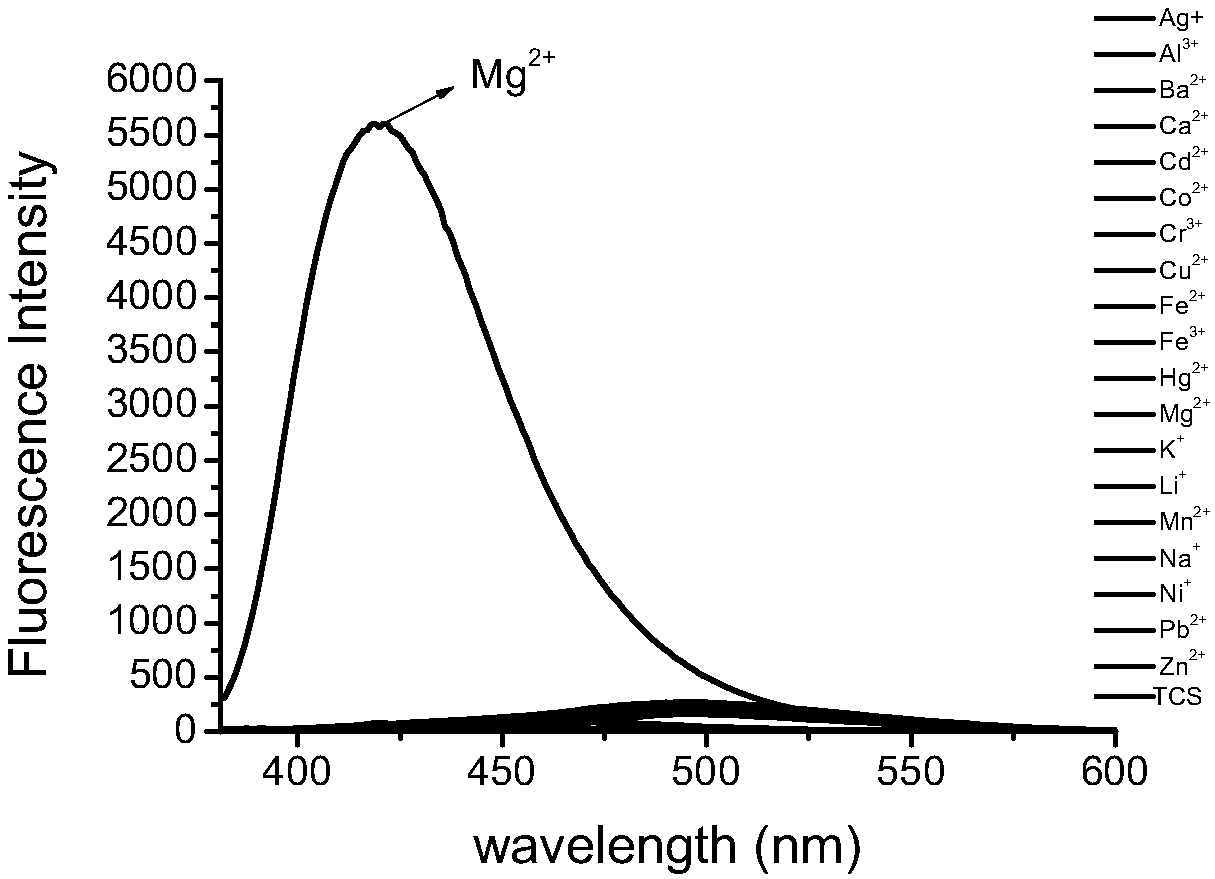
A fluorescent probe and coumarin-based technology, applied in the field of fluorescent probes, can solve problems such as cardiac arrest, shortness of breath, and prevention of calcium ion absorption, and achieve the effects of low detection limit, high sensitivity, and high anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1), the reaction formula of synthetic 7-hydroxyl-8-coumarin aldehyde:
[0031]
[0032] (2), the specific steps of synthetic 7-hydroxyl-8-coumarin aldehyde:
[0033] Dissolve 7-hydroxycoumarin and hexamethylenetetramine in glacial acetic acid, stir and heat to 70-100°C for reaction, then add hydrochloric acid to the system and stir at 50-90°C; cool, add ice water, Extract with ethyl acetate, then dry the organic phase with anhydrous sodium sulfate, filter under reduced pressure, and remove the solvent by rotary evaporation of the filtrate under reduced pressure to obtain a yellow solid; then recrystallize with absolute ethanol to obtain 7-hydroxy-8-coumarin Urine, the yield is about 18%.
[0034] (3), the reaction formula of synthetic coumarin fluorescent probe:
[0035]
[0036] (4), the specific steps of synthetic coumarin fluorescent probe:
[0037] In a 100ml three-necked flask, the intermediate 7-hydroxyl-8-coumarin aldehyde (0.400g, 2.1mmol) was dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com