A kind of phenolphthalein Schiff base fluorescent probe and its preparation method and application
A fluorescent probe, Schiff base technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex synthesis methods, low fluorescence quantum yield, etc., and achieve high detection sensitivity and high anti-interference. Ability, the effect of low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
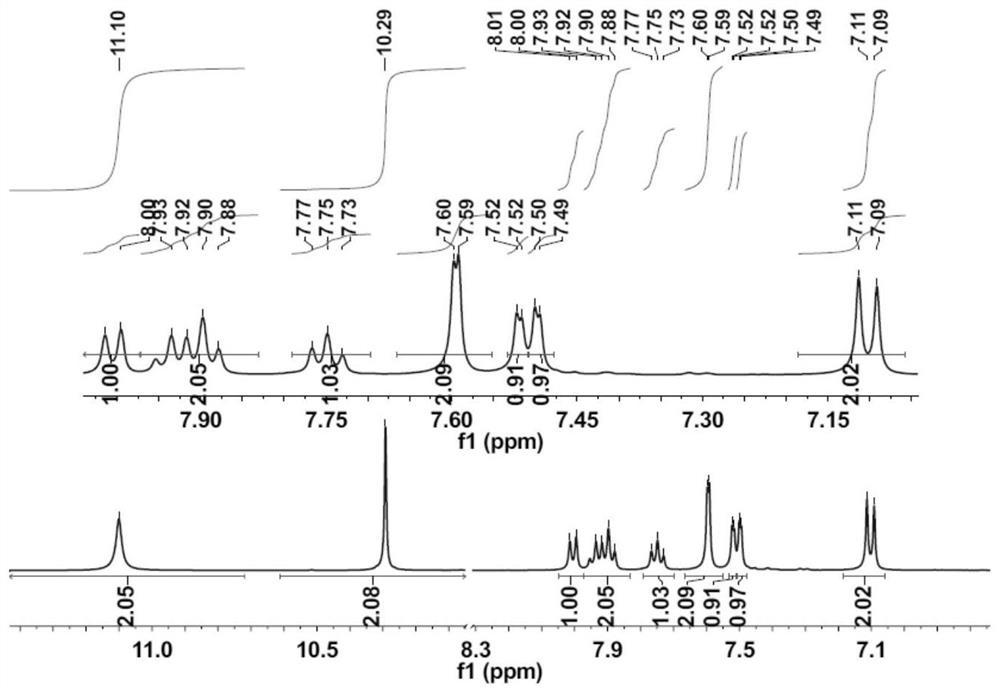
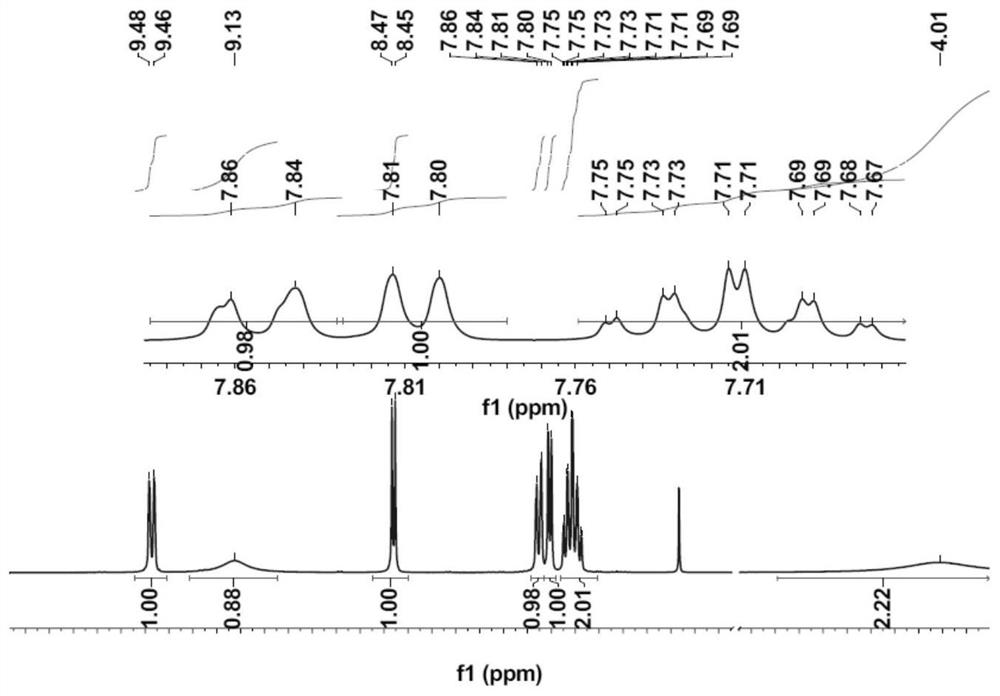
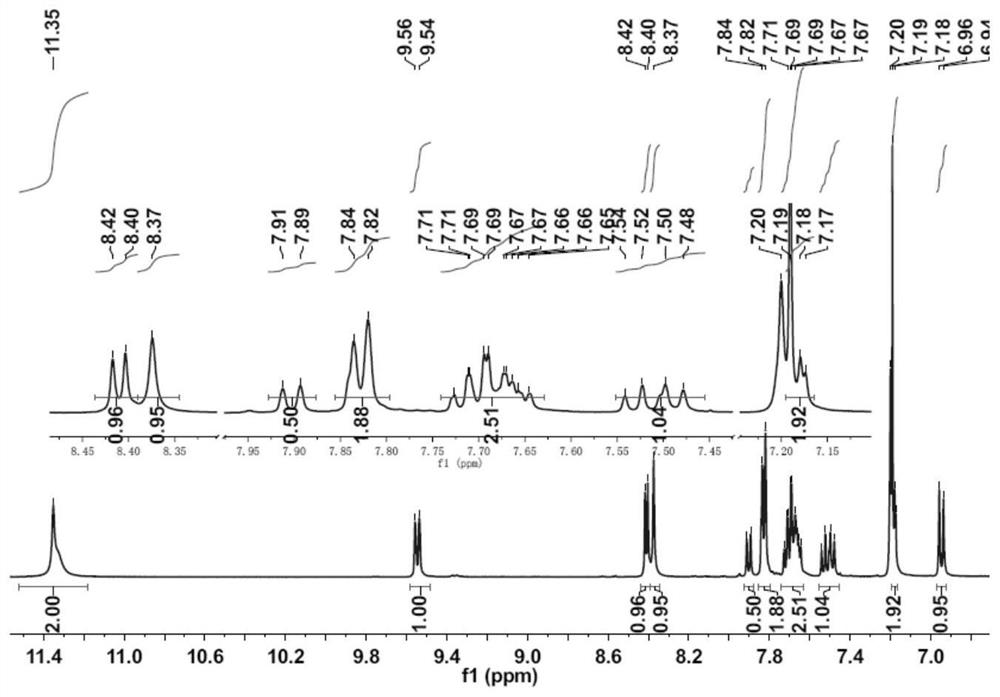
[0033] A kind of phenolphthalein Schiff base fluorescent probe of the present invention, concrete structural formula is as follows:
[0034]
[0035] The above-mentioned phenolphthalein Schiff base fluorescent probe can be applied to identify Al in DMSO solvent 3+ .
[0036] The preparation method of the phenolphthalein Schiff base fluorescent probe comprises the following steps:
[0037] S1. Synthesis of phenolphthalein dialdehyde: Dissolve 2.5466g (8mmol) of phenol and 1.6148g (11.5mmol) of hexamethylenetetramine in 40mL of trifluoroacetic acid, stir and heat to 70°C and react for 8h, then rotary evaporate to remove excess Add 60mL of water, stir for 30mins under heating; then cool in an ice bath, a white solid is precipitated, which is phenolphthalein dialdehyde, yield: 1.30g.
[0038] S2. Synthesis of isoquinoline hydrazide: prepare isoquinoline hydrazide by one-pot method, dissolve 3.2g (18.5mmol) isoquinoline carboxylic acid in 180mL methanol, slowly add 8mL of conc...
Embodiment 2
[0052] The preparation method of the phenolphthalein Schiff base fluorescent probe comprises the following steps:
[0053] S1. Synthesis of phenolphthalein dialdehyde: 2.5466g (8mmol) phenolphthalein and 1.3458g (9.6mmol) hexamethylenetetramine were dissolved in 35mL trifluoroacetic acid, stirred and heated to 95°C and reacted for 7h, then rotary evaporated to remove excess Trifluoroacetic acid; add 60mL of water, heat at 60°C, stir for 30mins; cool in an ice bath, a white solid is precipitated, which is phenolphthalein dialdehyde, yield: 1.15g;
[0054] S2. Synthesis of isoquinoline hydrazide: One-pot method was used to prepare isoquinoline hydrazide, 3.4736 g (20 mmol) of isoquinoline carboxylic acid was dissolved in 185 mL of methanol, 8 mL of concentrated sulfuric acid was slowly added dropwise, stirred and heated to 80°C After reacting for 18 hours, cooling in an ice bath, adding saturated sodium bicarbonate solution, extracting the solution with dichloromethane, taking t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com