Fusion protein with immune regulation function, pharmaceutical composition, cell and application
A fusion protein and cell technology, applied in the field of immune enhancer, cell and its application, and pharmaceutical composition, can solve the problem of insufficient response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] This example is for the preparation of DNA and mRNA encoding antigens and fusion proteins
[0069] 1. Preparation of DNA and mRNA Constructs
[0070] Construction of a DNA sequence for encoding CMV viral pp65 protein mRNA (sequence shown in SEQ ID NO: 7), DNA sequence for encoding sPD-1-Fc, sCD80-Fc and sPD-1-Fc-CD80 fusion protein mRNA , and used for subsequent in vitro transcription reactions. Constructs were prepared by codon optimization to introduce a high GC sequence to stabilize the synthesized mRNA, followed by a 3'UTR sequence derived from human β-globin, followed by a polyadenosine fragment. The specific sequence is shown in Table 1.
[0071] Table 1
[0072] name nucleic acid sequence number sPD-1 SEQ ID NO: 1 sCD80 SEQ ID NO: 2 Fc SEQ ID NO: 3 PD1-Fc-CD80 SEQ ID NO: 4
[0073] 2. In vitro transcription
[0074] The prepared corresponding DNA plasmid was firstly linearized by using speI endonuclease, and using ...
Embodiment 2
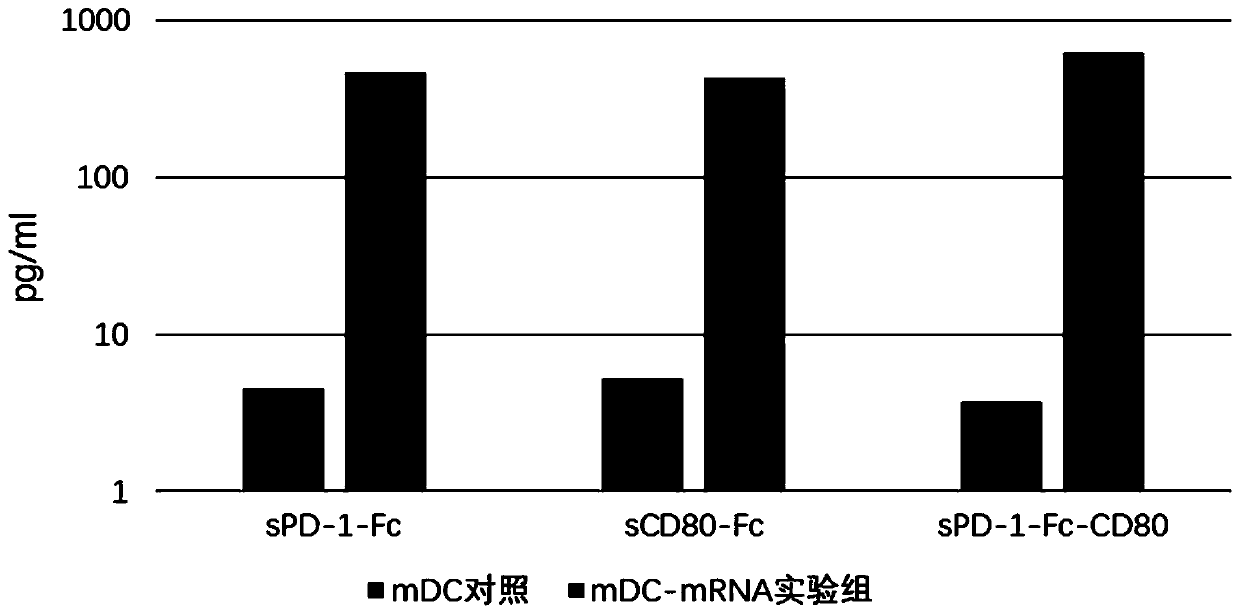
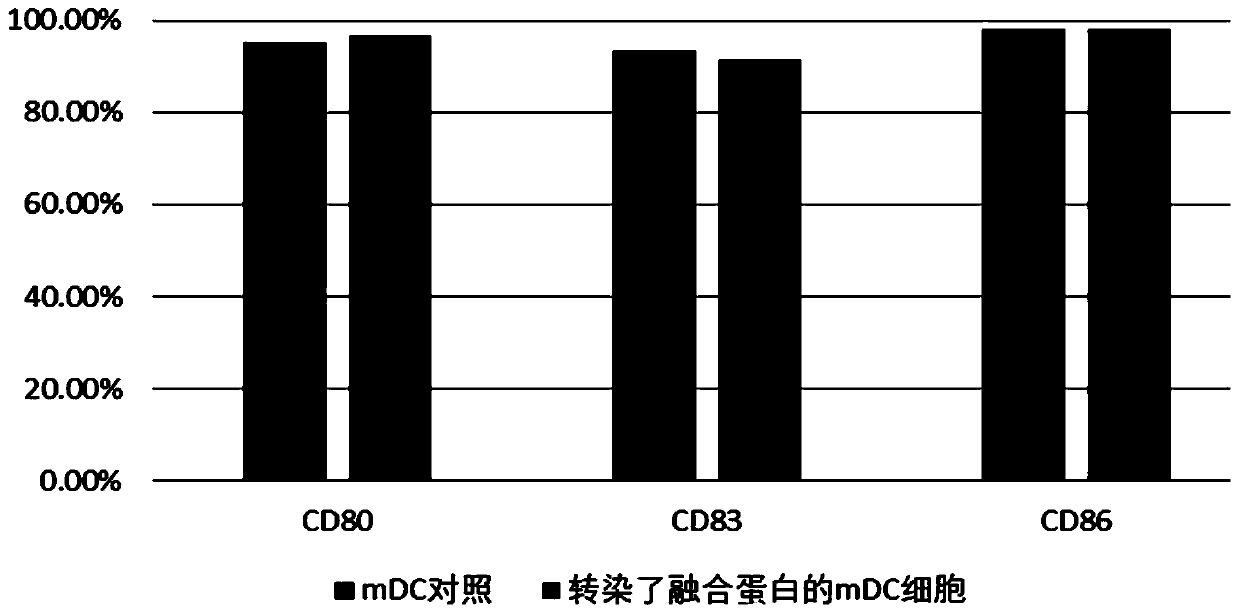
[0076] This example is used to verify the expression level of the fusion protein mRNA of the present invention in dendritic cells and its influence on the phenotype of dendritic cells.
[0077] 1. Induction culture of DC cells in vitro
[0078] Aseptically extract 50ml of human venous blood, separate peripheral blood mononuclear cells with lymphocyte separation medium in the ultra-clean workbench, add the mononuclear cells to the AIM-V medium, and place them in 37 ° C, 5% CO 2 Incubate in an incubator to allow monocytes to adhere to the wall. After 2h, the non-adherent cells were removed, and the adherent cells were added to iDC medium (GM-CSF with a final concentration of 800U / mL and IL-4 at 500U / mL were added to the AIM-V medium), and placed at 37°C for 5 %CO 2Cultured in the incubator for 6 days. Transfer half of the cell culture medium to a centrifuge tube, collect the cells by centrifugation at 500g, remove the supernatant, add an equal volume of fresh mDC medium, and ...
Embodiment 3
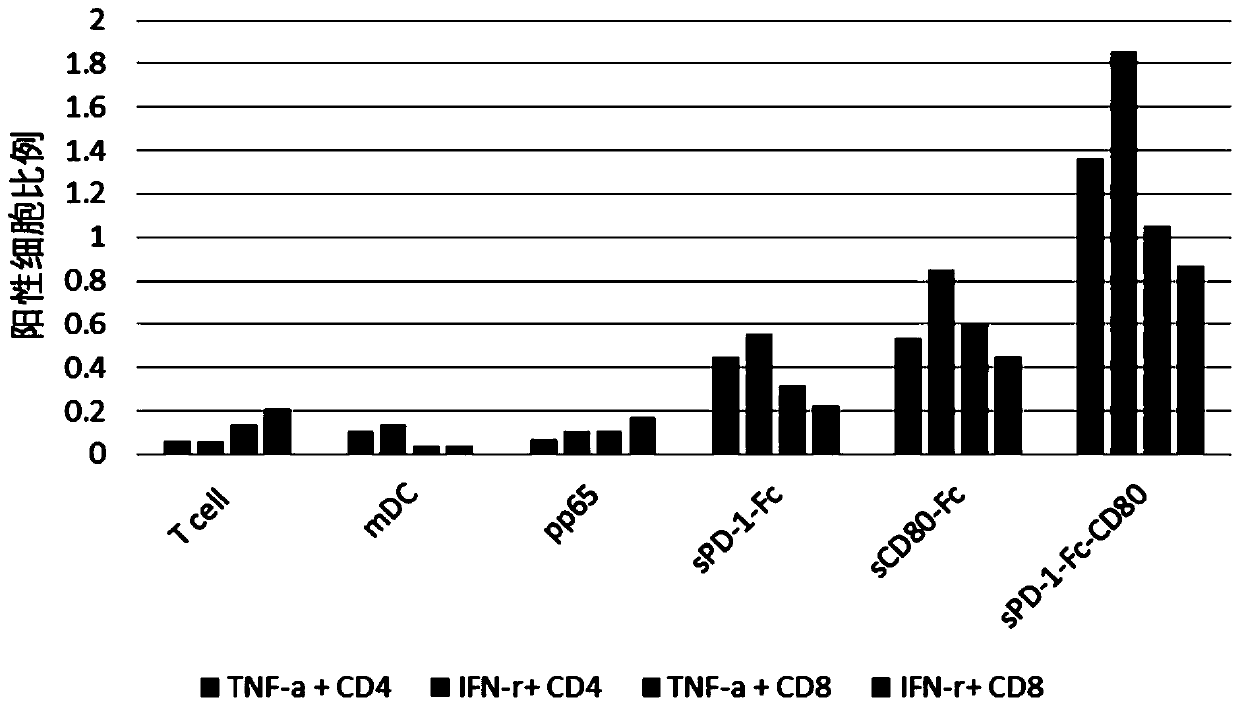
[0088] This example is used to study the influence of immunomodulator composition on T cell response
[0089] 1. Induction culture of DC cells in vitro
[0090] Aseptically extract 50ml of human venous blood, separate peripheral blood mononuclear cells with lymphocyte separation medium in the ultra-clean workbench, add the mononuclear cells to the AIM-V medium, and place them in 37 ° C, 5% CO 2 Incubate in an incubator to allow monocytes to adhere to the wall. After 2h, the non-adherent cells were removed, and the adherent cells were added to iDC medium (GM-CSF with a final concentration of 800U / mL and IL-4 at 500U / mL were added to the AIM-V medium), and placed at 37°C for 5 6 days in a % CO2 incubator. Transfer half of the cell culture medium to a centrifuge tube, collect the cells by centrifugation at 500g, remove the supernatant, add an equal volume of fresh mDC medium, and its formula is 1600U / mL GM-CSF and 1000U / mL IL-4, TNF-a( 5ng / ml), IL-1β(5ng / ml), IL-6(150ng / ml) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com