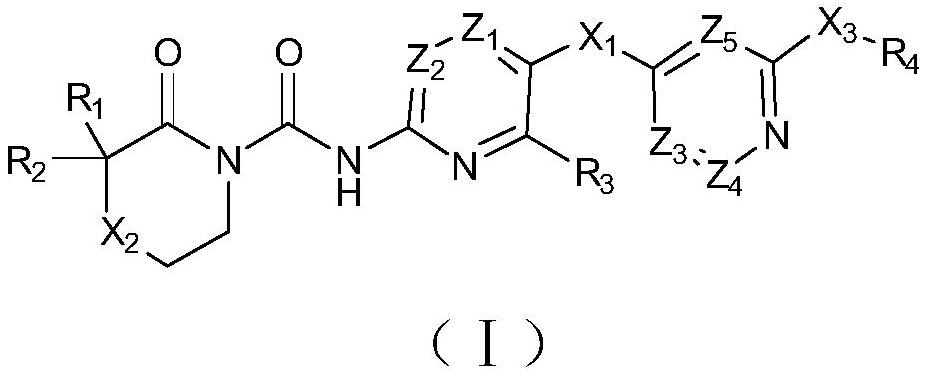
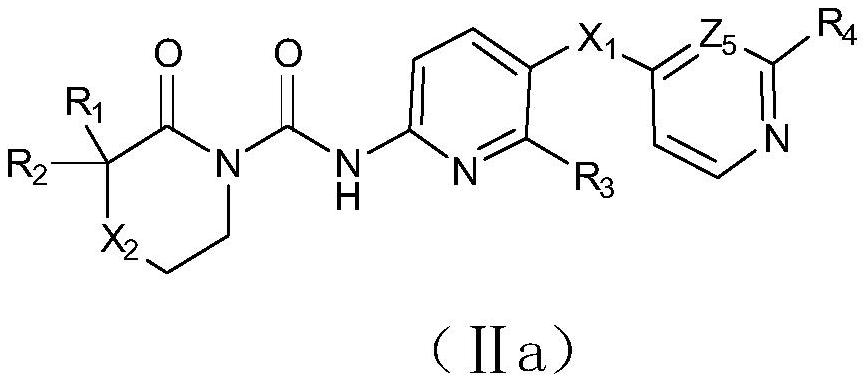
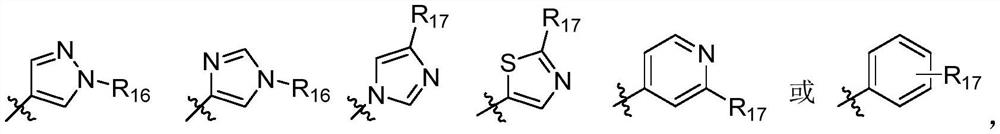
N-(azaaryl) cyclic lactam-1-carboxamide derivative and its preparation method and application
A technology of heteroaryl and aryl, applied in the field of N-cyclic lactam-1-carboxamide derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0162] 1. Preparation of intermediates
[0163] Intermediate A1: 1-(2-methoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H- Preparation of pyrazole
[0164]
[0165] 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (582 mg, 3.0 mmol) dissolved in N,N-dimethyl Dimethylformamide (5 mL), 1-bromo-2-methoxyethane (798 mg, 6.0 mmol), cesium carbonate (2.9 g, 9.0 mmol) and sodium iodide (224 mg, 1.5 mmol) were added. The reaction was stirred at 70° C. for 3 hours, then poured into water (50 mL), extracted with ethyl acetate (40 mL×2), the organic phases were combined and washed with brine (40 mL), dried over sodium sulfate and filtered, and the filtrate was concentrated to give 1-(2 -Methoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole crude (240 mg, yield 32%). MS m / z(ESI):253[M+H] + .
[0166] Intermediate A2: 4-(2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl ) Preparation of ethyl) morpholine
[0167]
...
Embodiment 1
[0424] Example 1: 3,3-Dimethyl-N-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxo) Preparation of pyridin-2-yl)-2-carbonylpyrrolidine-1-carboxamide
[0425]
[0426] The first step: preparation of 3,3-dimethyl-2-carbonylpyrrolidine-1-carbonyl chloride
[0427]
[0428] In an ice bath, a solution of 3,3-dimethylpyrrolidin-2-one (200mg, 1.75mmol) and pyridine (415mg, 5.26mmol) in dichloromethane (5mL) was dropped into triphosgene (172mg, 0.58 mmol) in dichloromethane (5 mL). The reaction was stirred at 5°C for 30 minutes. The reaction solution was directly used in the next step without any treatment.
[0429] The second step: 3,3-dimethyl-N-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxo) Preparation of pyridin-2-yl)-2-carbonylpyrrolidine-1-carboxamide
[0430]
[0431]In an ice bath, a solution of 3,3-dimethyl-2-carbonylpyrrolidine-1-carbonyl chloride (0.33 mmol) in dichloromethane (10 mL) obtained in the previous step was dropped into 6-methy...
Embodiment 9
[0444] Example 9: N-(5-((2-(1H-pyrazol-4-yl)pyridin-4-yl)oxo)-6-methylpyridin-2-yl)-3,3-dimethyl Preparation of yl-2-carbonylpyrrolidine-1-carboxamide
[0445]
[0446] The first step: tert-butyl 4-(4-((6-(3,3-dimethyl-2-carbonylpyrrolidin-1-carboxalylamino)-2-methylpyridin-3-yl )Oxo)pyridin-2-yl)-1H-pyrazole-1-carboxylate
[0447]
[0448] Triphosgene (88mg, 0.296mmol) was dissolved in dichloromethane (2mL), and 3,3-dimethylpyrrolidin-2-one (100mg, 0.885mmol) and pyridine (209mg, 2.655mmol) were added under ice-cooling. Chloromethane (2 mL) solution and the reaction was stirred for 0.5 h under ice bath. It was then added to tert-butyl 4-(4-((6-amino-2-methylpyridin-3-yl)oxo)pyridin-2-yl)-1H-pyrazole-1 under ice bath - carboxylate (90 mg, 0.245 mmol) and pyridine (20 mg, 0.25 mmol) in dichloromethane (2 mL) and the reaction was stirred at room temperature for 1 hour. It was then poured into water (30mL), then extracted with dichloromethane (20mL×2), the organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com