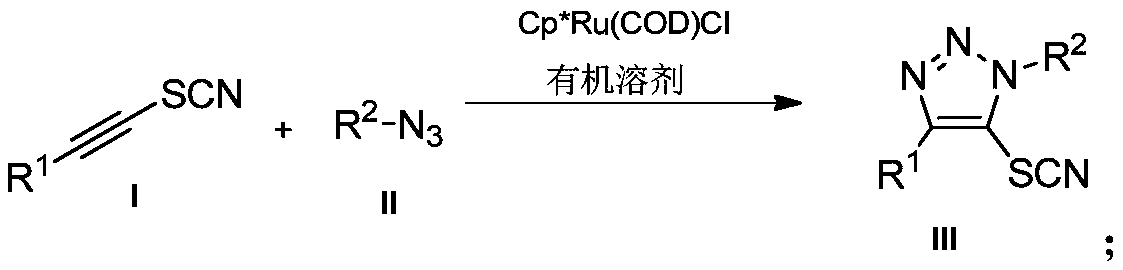
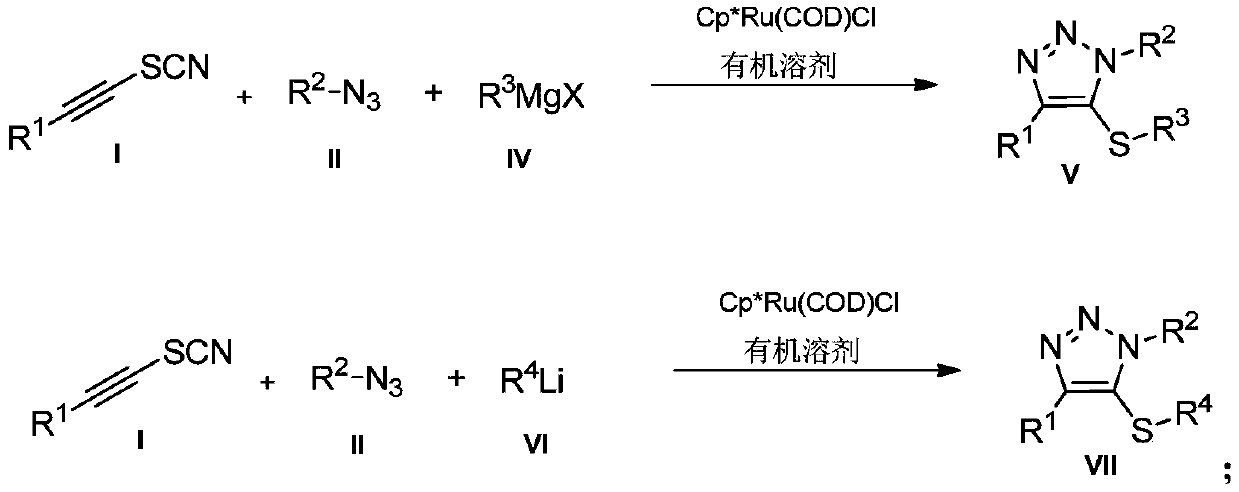
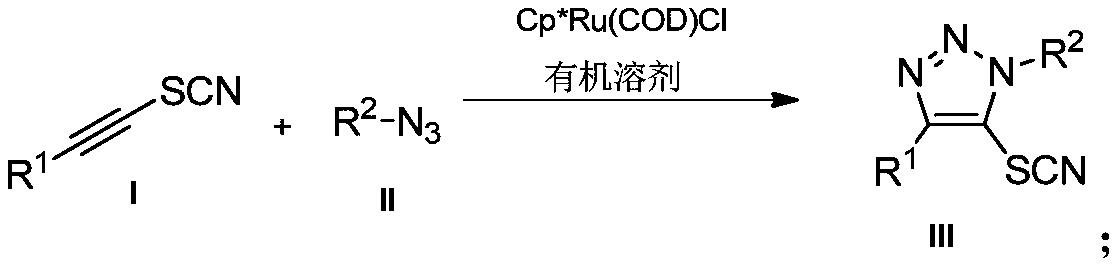
Preparation method and application of novel 5-thiocyanate substituted 1,4,5-trisubstituted 1,2,3-triazole
A thiocyanate, tri-substituted technology, applied in the field of preparation of 1,2,3-triazole, achieves the effects of mild reaction conditions and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 5-thiocyanate-(1-benzyl)-4-phenyl-1H-1,2,3-triazole
[0031] Under air, 1-phenylethynyl thiocyanate (0.2mmol, 32.0mg) was dissolved in tetrahydrofuran (2mL), and benzyl azide (0.3mmol, 40.2mg) and [Cp*Ru(COD) Cl] (0.005mmol, 1.9mg), the reaction mixture was stirred at room temperature, reacted for 12h, spin-dried and separated by column chromatography to obtain 48mg of white solid product with a yield of 82%.
[0032]
[0033] Mp=88-90℃.. 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.98-7.95(m,2H),7.55-7.48(m,3H),7.42-7.37(m,5H),5.80(s,2H). 13 C NMR (100MHz, CDCl 3 ): δ152.0, 133.4, 129.7, 129.3, 129.1, 129.0, 128.5, 127.9, 127.7, 112.3, 106.2, 53.6. HRMS (ESI-TOF) m / z calcd for C 16 h 12 N 4 S(M+Na) + 315.0674,found 315.0679.
Embodiment 2
[0034] Example 2: Preparation of 5-thiocyanate-(1-benzyl)-4-p-methoxyphenyl-1H-1,2,3-triazole
[0035] Under air, 1-p-methoxyphenylethynyl thiocyanate (0.2mmol, 37.8mg) was dissolved in tetrahydrofuran (2mL), and benzyl azide (0.3mmol, 40.2mg) and [Cp* Ru(COD)Cl] (0.005mmol, 1.9mg), the reaction mixture was stirred at room temperature, reacted for 12h, spin-dried and separated by column chromatography to obtain 50mg of white solid product with a yield of 78%.
[0036]
[0037] Mp=132-134°C. 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.93(d,J=8.0Hz,2H),7.40-7.37(m,3H),7.05(d,J=8.0Hz,2H),5.79(s,2H),3.89(s, 3H). 13 C NMR (100MHz, CDCl 3 ): δ160.8, 151.9, 133.5, 129.3, 129.1, 127.9, 121.0, 114.4, 111.3, 106.4, 55.4, 53.5. HRMS (ESI-TOF) m / z calcd for C 17 h 14 N 4 OS(M+Na)+ 345.0781,found 345.0784.
Embodiment 3
[0038] Example 3: Preparation of 5-thiocyanate-(1-benzyl)-4-p-methylphenyl-1H-1,2,3-triazole In air, 1-p-methyl Phenylethynyl thiocyanate (0.2mmol, 34.6mg) was dissolved in tetrahydrofuran (2mL), then benzyl azide (0.3mmol, 40.2mg) and [Cp*Ru(COD)Cl] (0.005mmol, 1.9 mg), the reaction mixture was stirred at room temperature, reacted for 12 h, spin-dried and separated by column chromatography to obtain 47 mg of a white solid product with a yield of 77%.
[0039]
[0040] Mp=57-59°C. 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.83(d,J=8.0Hz,2H),7.40-7.32(m,5H),7.31(d,J=8.0Hz,2H),5.78(s,2H),2.41(s, 3H). 13 C NMR (100MHz, CDCl 3 ): δ154.6, 147.3, 134.2, 132.8, 130.3, 129.2, 129.1, 129.1, 128.9, 128.5, 127.9, 53.1, 21.9. HRMS (ESI-TOF) m / z calcd for C 17 h 14 N 4 S(M+Na) + 329.0831,found 329.0835.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com