Daidzein group-containing main chain type benzoxazine and preparation method thereof
A technology of benzoxazine and daidzein, applied in the field of daidzein-based main chain type benzoxazines and preparation thereof, can solve the problems of unreported preparation and performance of benzoxazine resin, and achieve good environmental friendliness characteristics, broaden the scope of application, the effect of excellent comprehensive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of daidzein-containing main chain type benzoxazine:
[0035] S1. Weigh 5.09g (0.02mol) of daidzein and 3.97g (0.02mol) of 4,4'-diaminodiphenylmethane, and add them respectively to a reaction flask equipped with a stirrer, a thermometer and a condenser;
[0036] S2. Add 2.40 g (0.08 mol) of paraformaldehyde to S1, heat to 120° C., react for 16 hours, stop the reaction to obtain a mixed solution;
[0037] S3. The mixed solution obtained in S2 was suspended to remove the solvent, and then the product was dried in a vacuum oven for 12 hours to obtain 8.72 g of a yellow solid product with a yield of 87%.
[0038] The chemical reaction equation is as follows:
[0039]
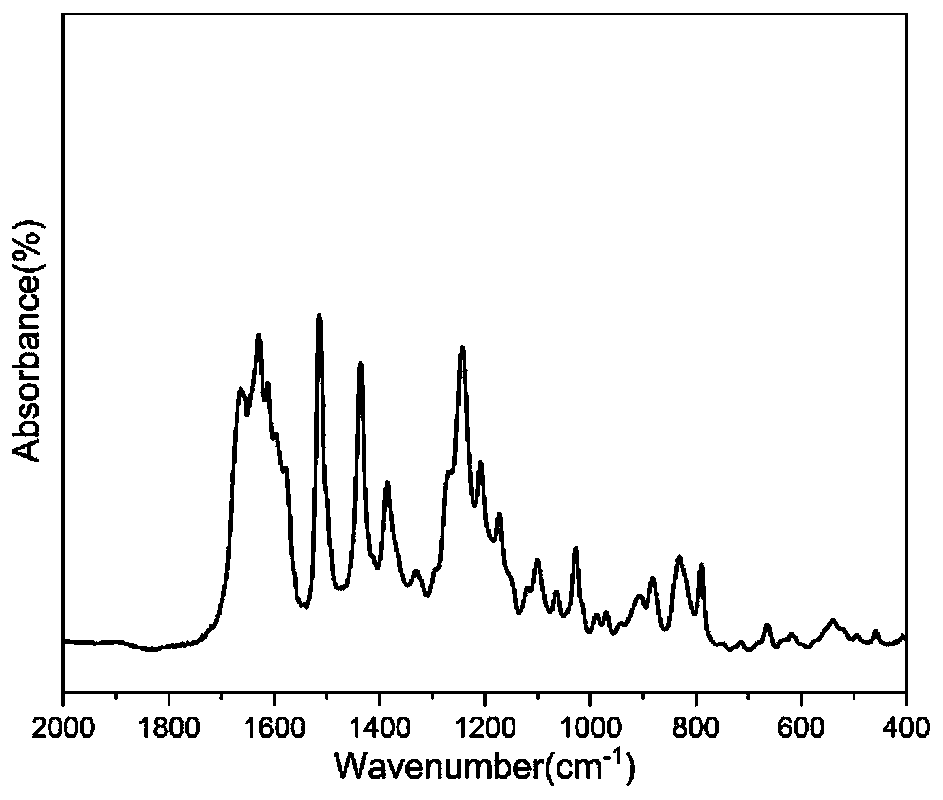
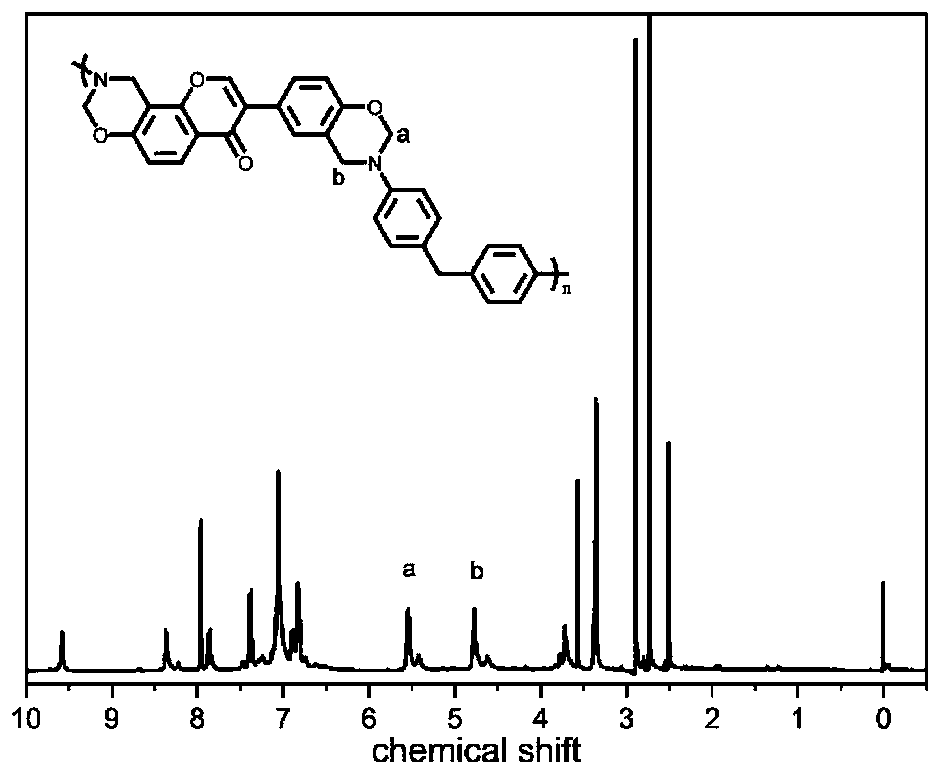
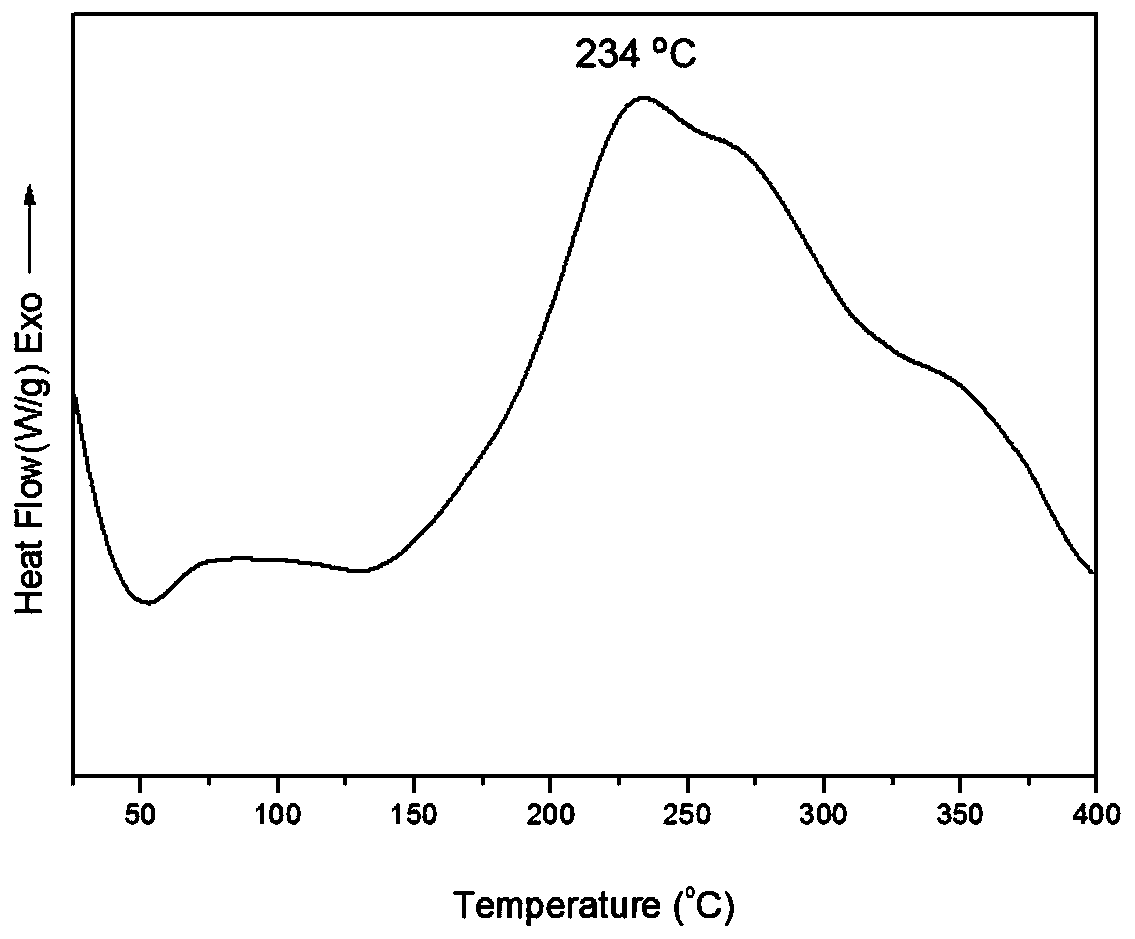
[0040] The TGA characterization results of the Fourier infrared spectrum, hydrogen nuclear magnetic resonance spectrum, DSC and cured resin of this product are shown in the appendix figure 1 , attached figure 2 , attached image 3 And attached Figure 4 . attached figure 1 It is an infra...
Embodiment 2
[0042] Preparation of main-chain benzoxazines containing daidzein groups: preparation of film materials
[0043] S1. Weigh 5.09g (0.02mol) of daidzein and 2.32g (0.02mol) of 1,6-hexamethylenediamine, and add them to a reaction flask equipped with a stirrer, a thermometer and a condenser respectively;
[0044] S2. Add 2.40 g (0.08 mol) of paraformaldehyde to S1, heat to 120° C., react for 16 hours, stop the reaction to obtain a mixed solution;
[0045] S3. The mixed solution obtained in S2 was suspended to remove the solvent, and then the product was dried in a vacuum oven for 12 hours to obtain 7.45 g of a yellow solid product with a yield of 82%.
[0046] The chemical reaction equation is as follows:
[0047]
Embodiment 3
[0049] Preparation of main-chain benzoxazines containing daidzein groups: preparation of film materials
[0050] S1. Weigh 5.09g (0.02mol) of daidzein and 2.44g (0.02mol) of 2,4-diaminodiphenyltoluene, and add them to a reaction flask equipped with a stirrer, a thermometer and a condenser respectively;
[0051] S2. Add 2.40 g (0.08 mol) of paraformaldehyde to S1, heat to 120° C., react for 16 hours, stop the reaction to obtain a mixed solution;
[0052] S3. The mixed solution obtained in S2 was suspended to remove the solvent, and then the product was dried in a vacuum oven for 12 hours to obtain 7.22 g of a yellow solid product with a yield of 85%.
[0053] The chemical reaction equation is as follows:
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com