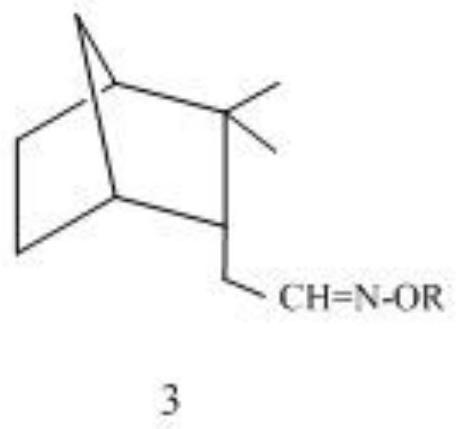
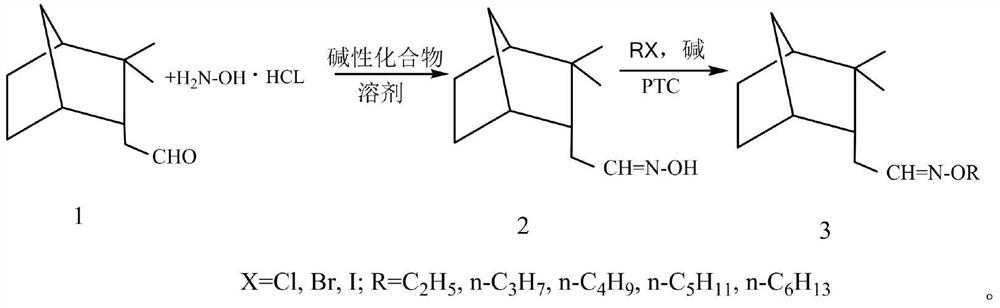
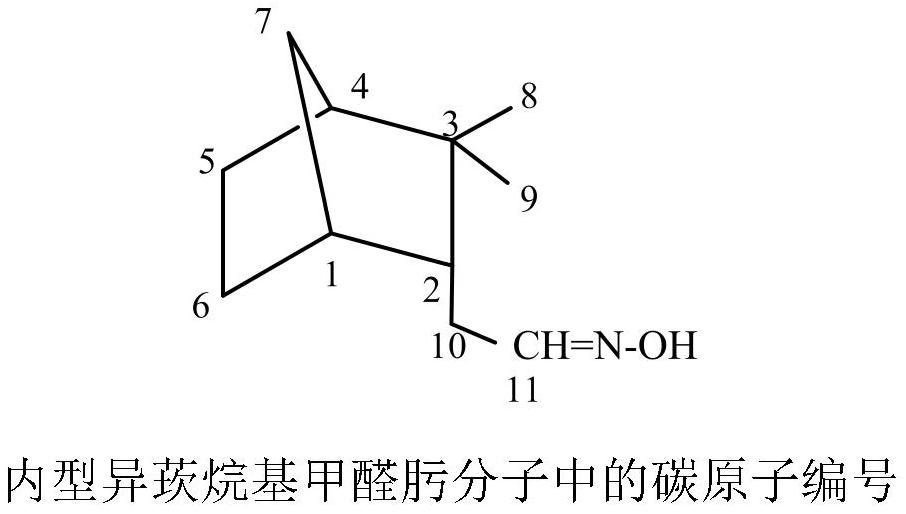
Synthesis method and antibacterial application of endo-isocamyl formaldehyde oxime and its alkyl ether
A technology of isobornyl and formaldehyde oxime is applied in the field of chemical synthesis of natural products, and can solve the problems such as reports on the synthesis and application of endo-isobornyl formaldehyde that have not yet been seen, and achieves high product yield, simple equipment and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of endo-isocamyl formaldehyde oxime, add 0.2 mol endo-isobornyl formaldehyde and 0.12 mol sodium bicarbonate / sodium carbonate / sodium acetate / sodium hydroxide / potassium hydroxide / triethyl ether in a 250mL Erlenmeyer flask Amine / pyridine, then add 40mL of 75% ethanol aqueous solution; dissolve 0.22mol or 0.24~0.26mol of hydroxylamine hydrochloride in 40mL of water, and slowly add it dropwise to the stirring endo-isocamyl formaldehyde solution through the dropping funnel, about 30 After the dropwise addition in 1 minute, heat the water bath to 50-60°C, take a sample after 8 hours, take the upper layer after standing, dilute it with petroleum ether, shake it up, remove the water in the lower layer, dry it with anhydrous sodium sulfate, and carry out gas chromatography analysis, if Endo-isocamyl formaldehyde can end the reaction. Ethanol and part of the water were evaporated under reduced pressure on a rotary evaporator, and extracted twice with petroleum ether (50...
Embodiment 2
[0025] Endo-isocamphoryl formaldehyde oxime ethyl ether (3a, R=C 2 h 5 ) synthesis, in a 150mL ground-mouth Erlenmeyer flask equipped with a reflux spherical condenser, add 0.02mol internal isobornyl formaldehyde oxime, 0.04mol bromoethane / chloroethane / iodoethane, 40mL benzene, 2.1 g40%Na0H aqueous solution and 0.0006mol tetra-n-butylammonium bromide were put into a magnetic stirrer, stirred and heated on a magnetic heating stirrer, and the heating temperature was 80-110°C until a small amount of reflux occurred, and a sample was taken after 4 hours. Gas chromatographic analysis followed the progress of the reaction. When there was no endo-isobornyl formaldehyde oxime in the reaction solution, stop heating, cool to room temperature under stirring, transfer the reaction solution into a separatory funnel, wash with saturated saline, Water and sodium sulfate are dried, the solvent is recovered by distillation, and the product is evaporated by vacuum distillation. Endo-isocampho...
Embodiment 3
[0028] Endo-isocamphoryl formaldehyde oxime n-propyl ether (3b, R=n-C 3 h 7 ) synthesis, in a 150mL ground-mouth Erlenmeyer flask equipped with a reflux spherical condenser, add 0.02mol internal isobornyl formaldehyde oxime, 0.04mol bromo-n-propane / chloro-n-propane / iodo-n-propane, 40mL Benzene, 2.1g of 40% NaOH aqueous solution and 0.0004mol of tetra-n-butylammonium iodide were put into a magnetic stirrer, stirred and heated on a magnetic heating stirrer, the heating temperature was 80-110°C, until a small amount of reflux occurred, 4 hours Then take a sample for gas chromatography analysis to track the progress of the reaction. When there is no endo-isocamyl formaldehyde oxime in the reaction solution, stop heating, cool to room temperature under stirring, transfer the reaction solution into a separatory funnel, and wash with saturated saline. , dried over anhydrous sodium sulfate, the solvent was recovered by distillation, and the product was evaporated by vacuum distillati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com