Method for manufacturing gel polymer electrolyte and gel-state battery through in-situ ring-opening polymerization
A technology of gel polymer and ring-opening polymerization, which is applied in the direction of secondary batteries, circuits, electrical components, etc., can solve the problems that affect the battery rate performance, battery capacity, high impedance at the interface, etc., to improve cycle and safety performance, and prevent corrosion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
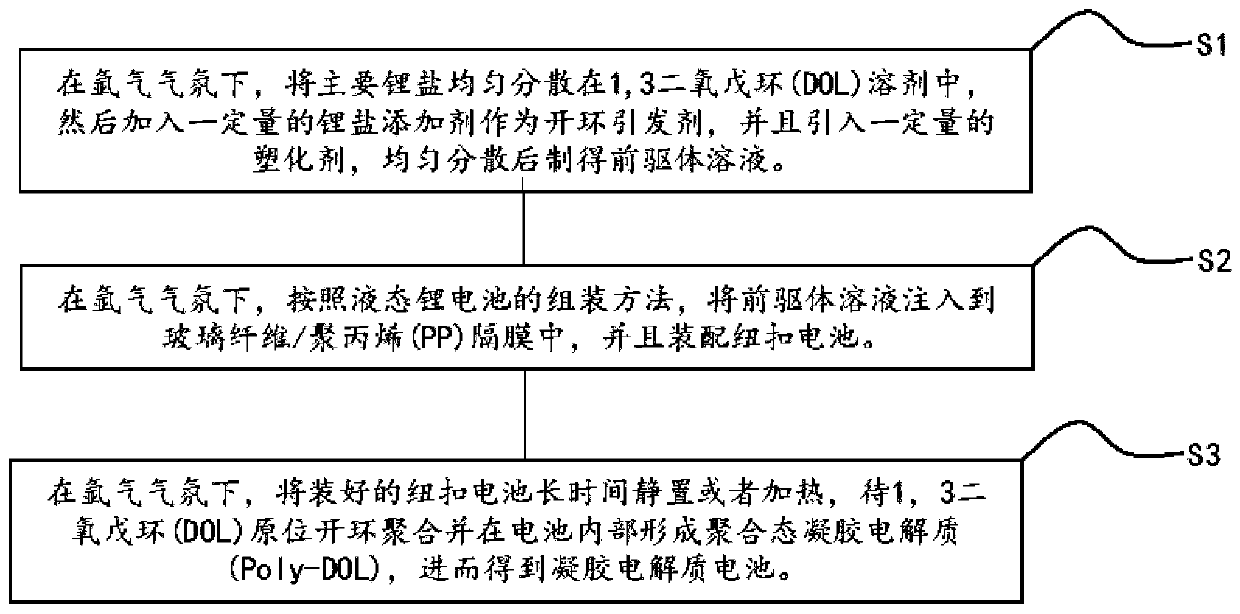
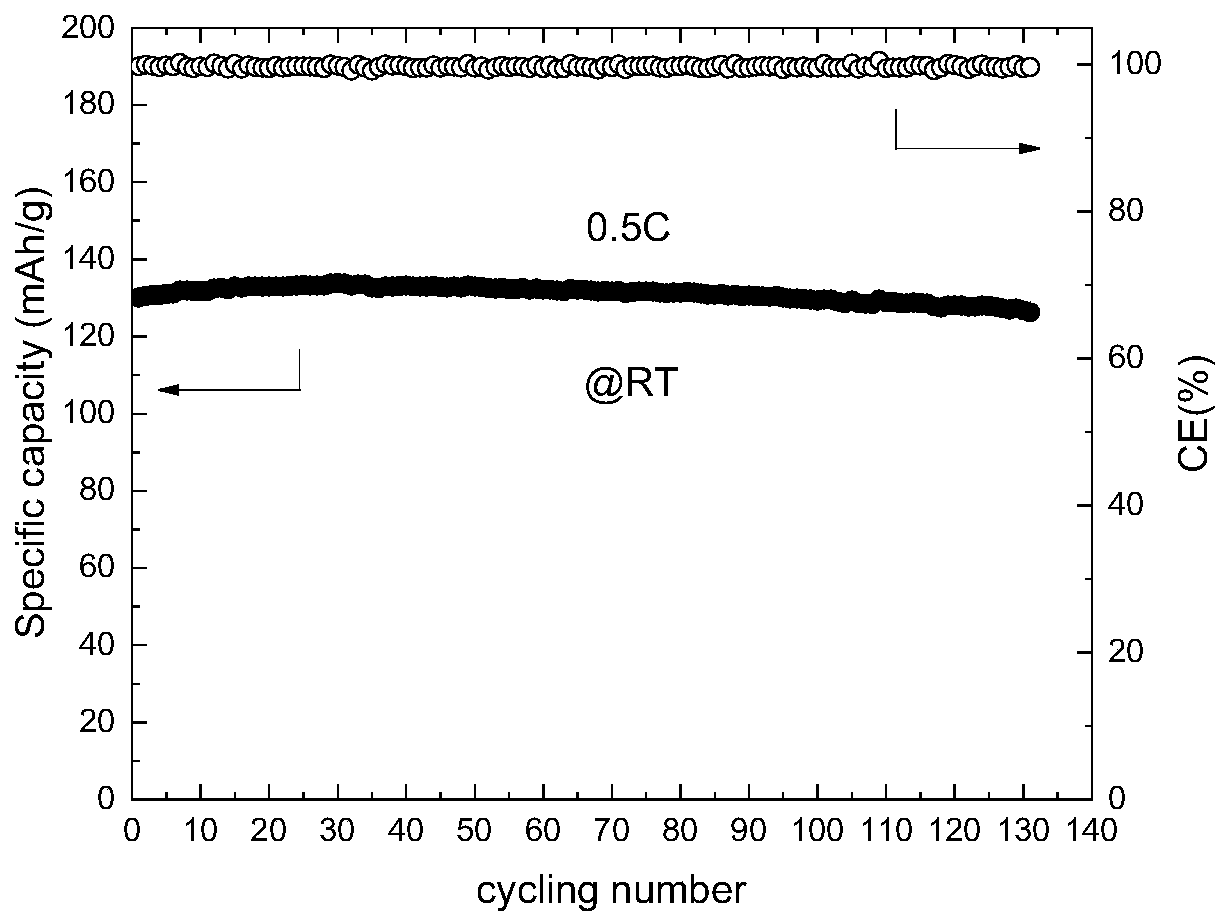
[0028] This embodiment is all carried out under an argon atmosphere. A certain amount of 1,3 dioxolane (DOL) solvent is added to a glass beaker, and then 1 mol / L of bis(trifluoromethylsulfonyl)imide lithium is added successively. (LiTFSI), 0.1mol / L lithium difluorooxalate borate (LiDFOB), stir evenly to obtain a precursor solution; then inject the precursor solution into the glass fiber, and assemble a stainless steel / / stainless steel button battery, and put the battery at room temperature After standing for 10 days, the in-situ ring-opening polymerization reaction was sufficient, and the lithium ion conductivity of the gel electrolyte at room temperature was measured to be 1.83×10 -6 S / cm.
Embodiment 2
[0030] This embodiment is all carried out under an argon atmosphere. A certain amount of 1,3 dioxolane (DOL) solvent is added to a glass beaker, and then 1 mol / L of bis(trifluoromethylsulfonyl)imide lithium is added successively. (LiTFSI), 0.3mol / L lithium difluorooxalate borate (LiDFOB), stir well to obtain a precursor solution; then inject the precursor solution into the glass fiber, and assemble a stainless steel / / stainless steel button battery, and put the battery at room temperature After standing for 5 days, until the in-situ ring-opening polymerization reaction is sufficient, the lithium ion conductivity of the gel electrolyte at room temperature is measured to be 3.29×10 -6 S / cm.
Embodiment 3
[0032] This embodiment is all carried out under an argon atmosphere. A certain amount of 1,3 dioxolane (DOL) solvent is added to a glass beaker, and then 1 mol / L of bis(trifluoromethylsulfonyl)imide lithium is added successively. (LiTFSI), 0.5mol / L lithium difluorooxalate borate (LiDFOB), stir well to obtain a precursor solution; then inject the precursor solution into the glass fiber, and assemble a stainless steel / / stainless steel button battery, and put the battery at room temperature After standing for 5 days, the in-situ ring-opening polymerization reaction was sufficient, and the lithium ion conductivity of the gel electrolyte at room temperature was measured to be 2.16×10 -6 S / cm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com