Application of chloroquine or derivative hydroxychloroquine
A derivative, the technology of hydroxychloroquine, applied in the field of biomedicine, can solve the problems of high price, limited wide application, and limited drugs, and achieve the effect of low price, saving social resources, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
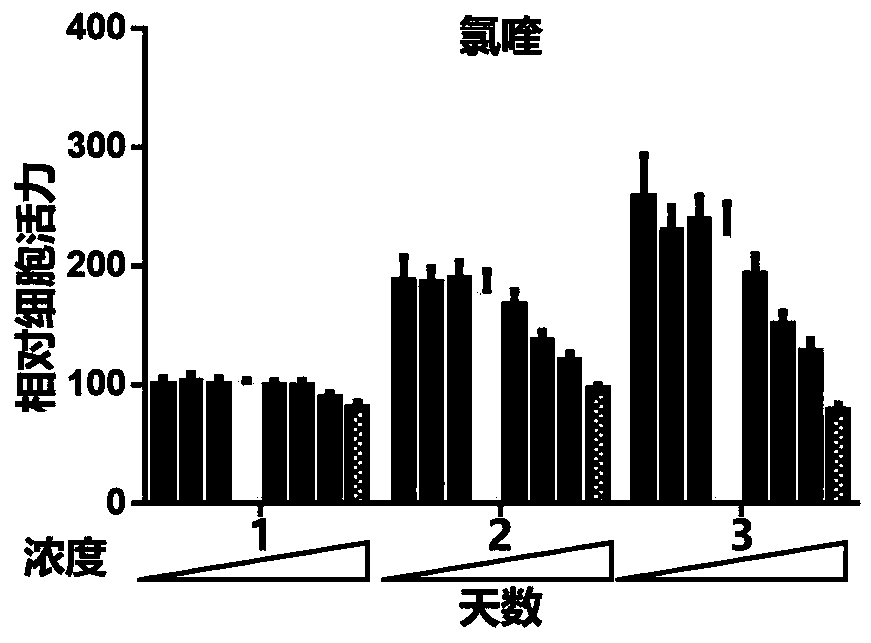
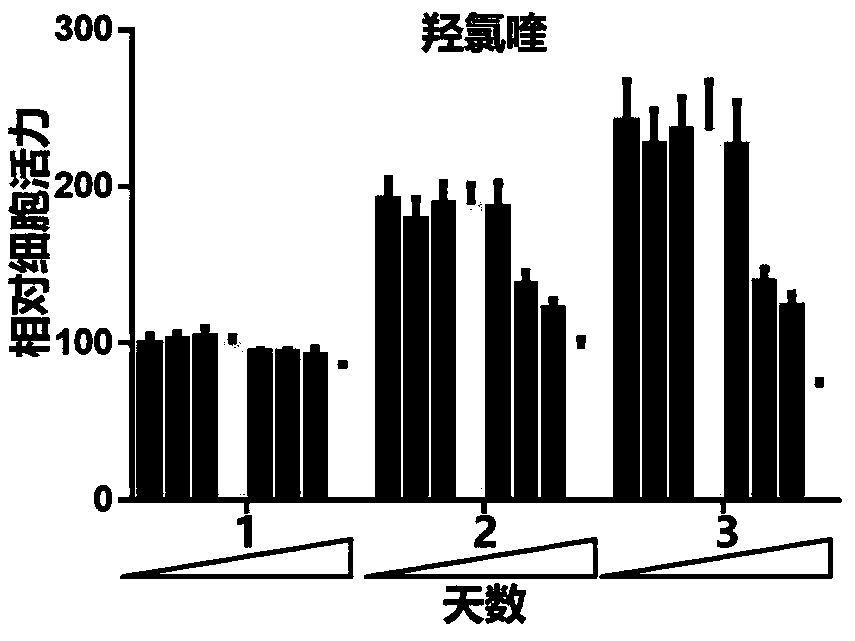
[0029] Example 1 Chloroquine and its derivative hydroxychloroquine inhibit the proliferation of OFs in patients with GO
[0030] Method steps:
[0031] A) OFs from GO patients (n=6) were extracted and passaged to P2 in proliferation medium for experiments. They were divided into chloroquine group (0, 0.5, 1, 5, 10, 25, 50, 100 μM) and hydroxychloroquine group (0, 0.5, 1, 5, 10, 25, 50, 100 μM) , 0.5, 1, 5, 10, 25, 50, 100 μM), after starting the treatment, add the same concentration of CCK8 for 3 consecutive days and read the absorbance (450 nm) after culturing for 4 h. As the concentration of chloroquine or hydroxychloroquine increases and the action time is prolonged, Cell viability gradually decreased; B) OFs (n=6) from GO patients were extracted, passaged to P2 in proliferation medium for experiments, and divided into hydroxychloroquine groups (0, 0.5, 1, 5, 10, 25, 50, 100 μM) , after starting the treatment, adding the same concentration of CCK8 for 3 consecutive days an...
Embodiment 2
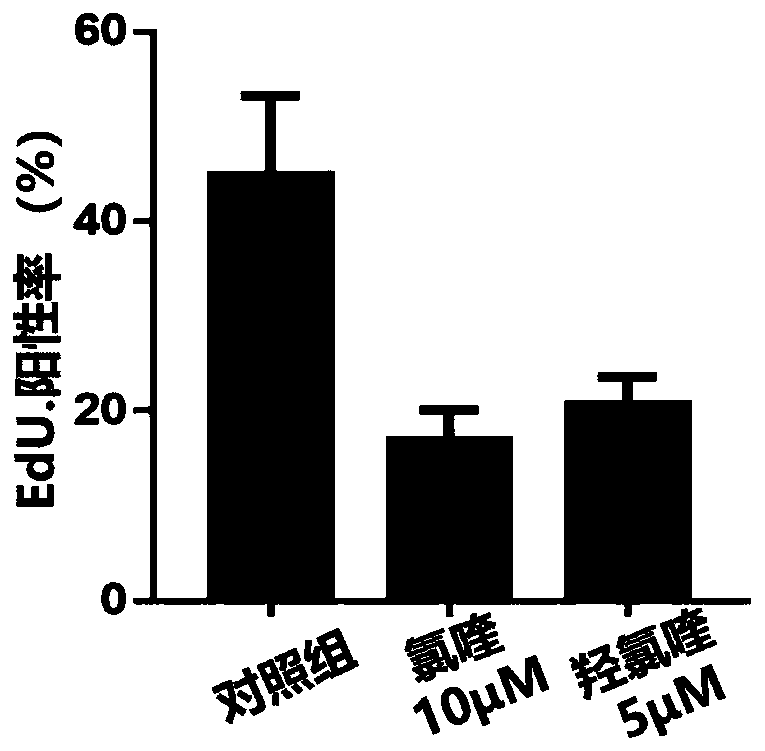
[0035] Example 2: Chloroquine and its derivative hydroxychloroquine inhibit the adipogenic differentiation of OFs in GO patients
[0036] Method steps:
[0037] A) The OFs (n=3) of GO patients were taken and passaged to P3 in the proliferation medium. When the cell density reached more than 90%, the induction medium was switched to. They were divided into the control group, the chloroquine group (10 μM) and the hydroxychloroquine group ( 5μM) in 3 groups in total, cells were fixed after 10 days, and normal phase contrast images and images after Oil Red O staining were taken with an optical microscope (100×).
[0038] B) After 4 days of successful induction and differentiation, the cells of each group were collected to extract RNA, and real-time quantitative PCR was performed to detect the changes of markers related to adipogenic differentiation.
[0039] result:
[0040] The exophthalmos of GO patients are mainly caused by the adipogenic differentiation of OFs compressing th...
Embodiment 3
[0042]Example 3: Chloroquine inhibits the synthesis and secretion of hyaluronic acid in OFs in GO patients
[0043] Method steps:
[0044] The OFs (n=4) of GO patients were taken and passaged in the proliferation medium to P3 for experiments. They were divided into IL-1β-treated group and non-treated group, and further divided into control group, chloroquine group (10 μM) and hydroxychloroquine group (5 μM). ) A total of 3 groups, treated cells for 48 hours, took the supernatant of the medium, centrifuged at 4°C to remove dead cells, and used a hyaluronic acid enzyme-linked immunosorbent assay (Enzyme-linked immunosorbent assay, ELISA) kit to detect the hyaluronic acid concentration. Cells were used to extract RNA to detect HAS2 expression.
[0045] result:
[0046] Hyaluronic acid is the main hydrophilic mucopolysaccharide in the orbit of GO patients, which promotes the increase of periorbital contents and compresses surrounding tissues, and its secretion is significantly i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com