Separation and purification method for acetylcysteine enantiomer
A technology of acetylcysteine and enantiomers, which is applied in the field of separation and purification of acetylcysteine enantiomers, can solve problems such as poor reproducibility, inaccurate quantification, and complicated separation and purification methods. Achieve accurate separation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0062] Example 1
[0063] 1. Apparatus and conditions:
[0064] High performance liquid chromatograph: Hitachi L2420;
[0065] Chromatographic column: cellulose coated chiral chromatographic column (model: Cellu-DR, 5μm, 4.6*250mm), the packing silica gel of this type of chromatographic column is coated with: cellulose-tris(3,5) dimethyl phenyl carbamate;
[0066] Mobile phase: n-hexane-isopropanol containing 0.5% acetic acid=80:20, the percentage is the volume percentage in isopropanol;
[0067] Flow rate: 1ml / min;
[0068] Column temperature: 25℃;
[0069] Injection volume: 20μL;
[0070] Detection wavelength: 220nm.
[0071] 2. Experimental methods and results:
[0072] (1) The preparation of the reference solution includes: accurately weigh the standard N-acetyl-L-cysteine and N-acetyl-D-cysteine, and prepare them with diluent (chromatographic grade absolute ethanol) The concentration is a reference solution containing 0.1, 0.2, 0.5, 1.0 mg N-acetyl-L-cysteine and 0.1, 0.2, 0.5, 1....
Example Embodiment
[0081] Example 2
[0082] Preparation of test solution 1: accurately weigh the standard N-acetyl-L-cysteine and N-acetyl-D-cysteine, and use the diluent (chromatographic grade absolute ethanol) to prepare the concentration to each 1ml contains 0.5mg N-acetyl-L-cysteine and 0.5mg N-acetyl-D-cysteine mixed solution, as the test solution 1.
[0083] Take the test solution 1 and test it according to the conditions in Example 1. Repeat the injection for 5 times. The peak area is shown in Table 2.
[0084] Table 2 Test product 1 five injection repeatability
[0085]
[0086]
[0087] Note: " / " means not detected or not calculated.
[0088] It can be seen from Table 2 that the detection method in this application has good repeatability.
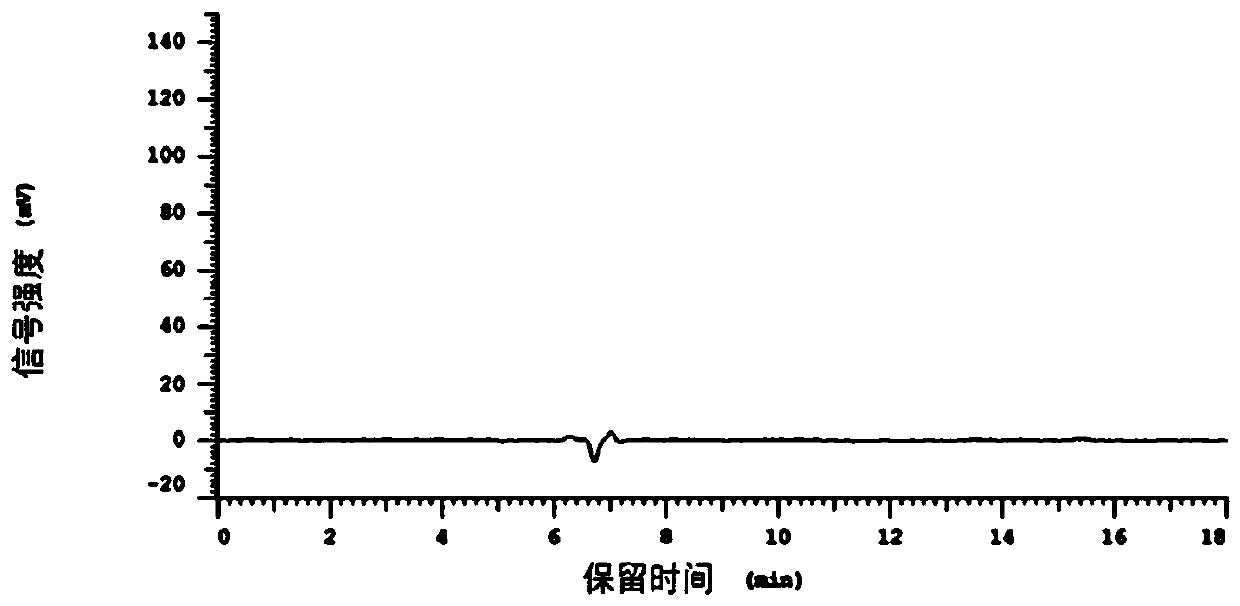
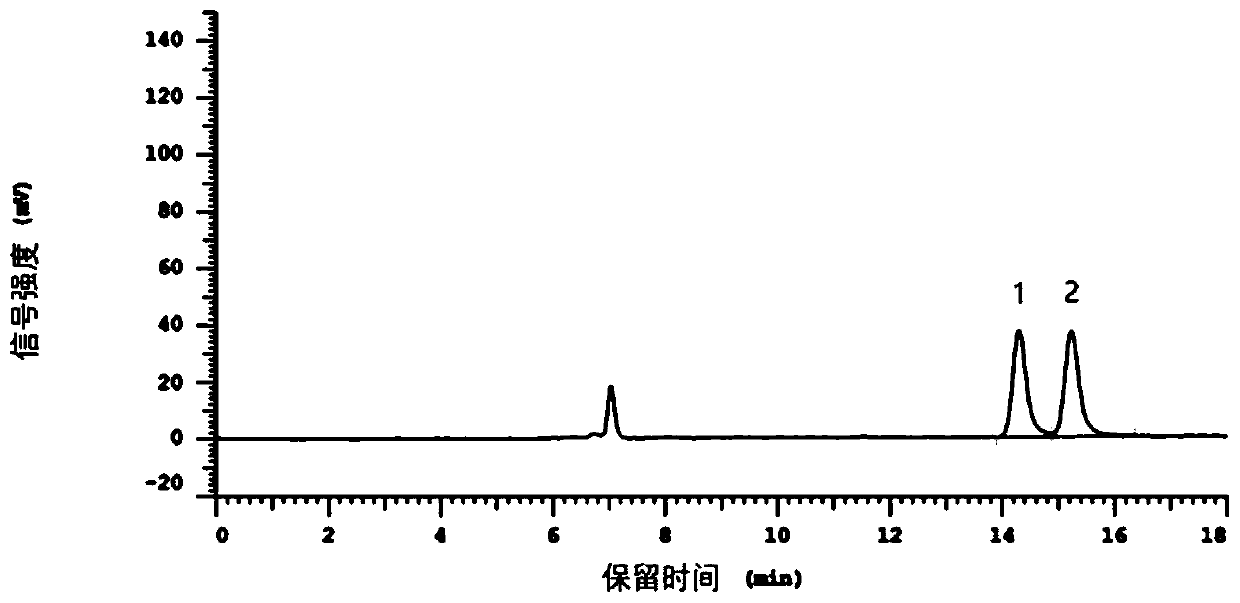
[0089] figure 1 This is the chromatogram of the solvent absolute ethanol in this example, Figure 2~6 It is the chromatogram of the first to fifth injections of the test solution 1.
[0090] by figure 2 It can be seen that the retention time of N-acetyl...
Example Embodiment
[0092] Example 3
[0093] Preparation of test solution 2: accurately weigh N-acetyl-DL-cysteine (synthesized from DL cysteine hydrochloride, and test the specific rotation as 0 according to AJI92 standard), and use diluent (chromatographic Grade absolute ethanol) as the test solution 2.
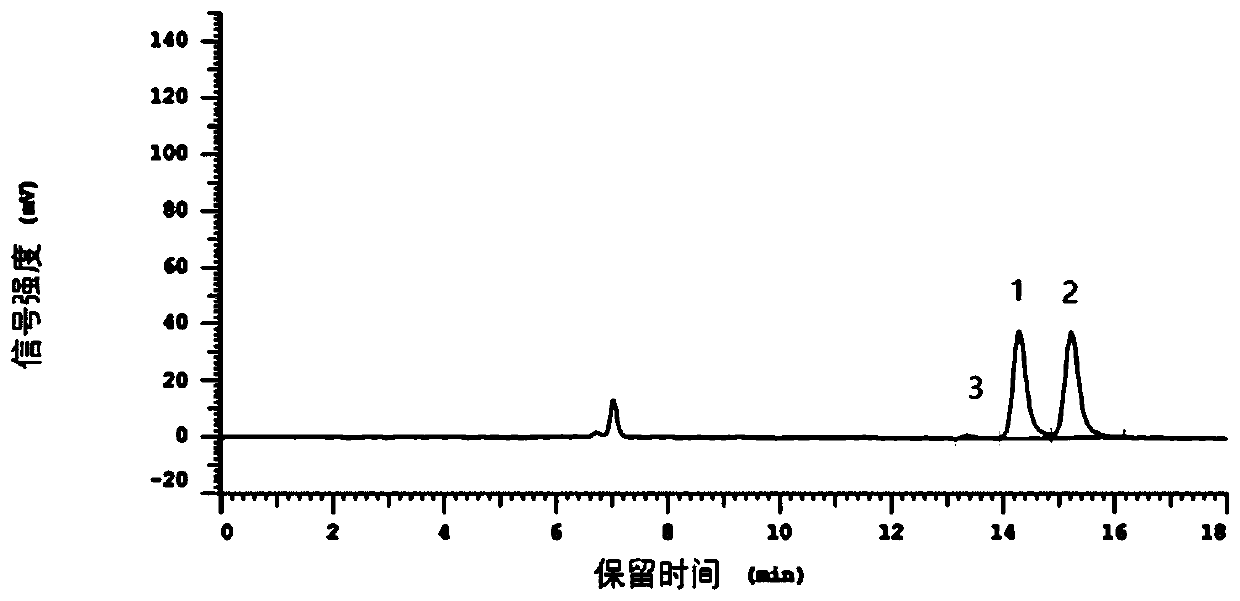
[0094] Take test solution 2 and test it according to the instrument and conditions in Example 1, and record the chromatogram. Such as Figure 7 Shown.
[0095] by Figure 7 Known:
[0096] (1) The retention time of N-acetyl-L-cysteine is 14.28 min, the peak area is 617334, the height is 35513, and the area% is 47.568%; the retention time of N-acetyl-D-cysteine is 15.21 min, peak area is 666171, height is 35225, area% is 51.331%; impurity peak retention time is 13.41min, peak area is 14298, height is 1100, area% is 1.102%; N-acetyl-L-cysteamine The resolution of acid and N-acetyl-D-cysteine is greater than 1.5, the peak shape is good, no tailing, no protruding;
[0097] (2) Under the condi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap