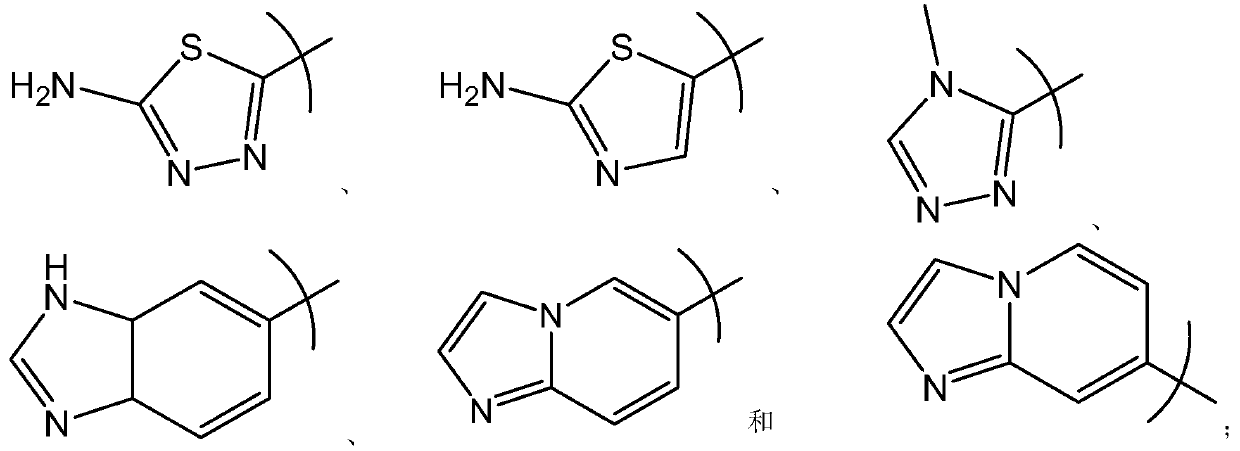
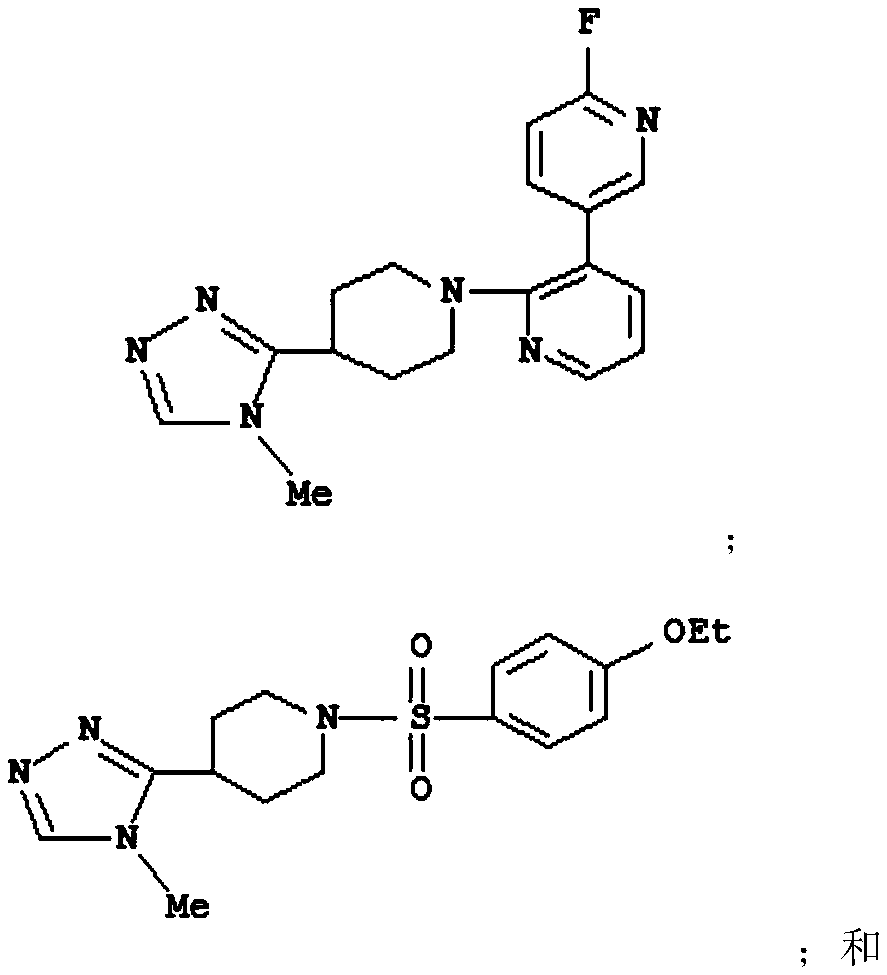
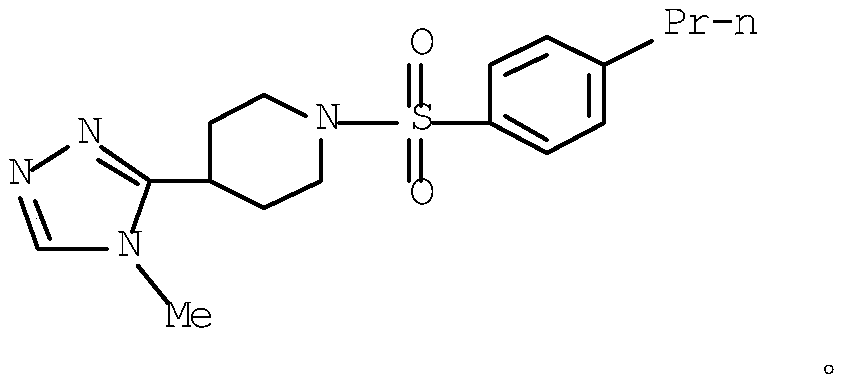
Novel inhibitors
A solvate, selected technology, applied in the directions of medical preparations containing active ingredients, allergic diseases, drug combinations, etc., can solve the problem of not showing sequence homology and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0960] In another embodiment, the present invention provides compounds of formula (IIa) and (IIb), wherein X 1 , n, Z, Y 1 , Y 2 , Y 3 , Y 4 , Y 5 , Y 6 , Y 7 , Y 8 , Y 9 , Y 10 , R 5 and R 6 As defined in Examples 1 to 265:
[0961]
[0962]
[0963]
[0964]
[0965]
[0966]
[0967]
[0968]
[0969] In another embodiment, the present invention provides compounds of formula (IIIa) and (IIIb), wherein X 1 , n, Y 1 , Y 2 , Y 3 , Y 4 , Y 5 , Y 6 , Y 7 , Y 8 , Y 9 , Y 10 , R 5 and R 6 As defined in Examples 266 to 443:
[0970]
[0971]
[0972]
[0973]
[0974]
[0975]
[0976] In another embodiment, the present invention provides compounds of formula (IVa) and (IVb), wherein X 1 , o, Z, Y 1 , Y 2 , Y 3 , Y 4 , Y 5 , Y 6 , Y 7 , Y 8 , Y 9 , Y 10 , R 5 and R 6 As defined in Examples 444 to 795:
[0977]
[0978] In both formulas (IVa) and (IVb), o is 0.
[0979]
[0980]
[0981]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com