Proteinaceous heterodimer and use thereof
A heterodimer, protein technology, applied in the direction of animal/human protein, immunoglobulin, anti-enzyme immunoglobulin, etc., can solve the problem that the yield of heterodimer protein cannot be further improved, and cannot be controlled or eliminated very effectively. Protein homodimers and other issues have achieved significant anti-tumor activity, long in vivo half-life, and high expression yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0405] Method for preparing protein heterodimer or protein mixture
[0406] In another aspect, the present application provides a method for producing a protein heterodimer or a protein mixture comprising a protein heterodimer, the method comprising (i) culturing the host of the present application under conditions that affect the expression of the protein heterodimer Cells, and (ii) harvesting of expressed protein heterodimers or protein mixtures containing expressed protein heterodimers (such as the protein mixtures of the present application).
[0407] In some embodiments, the method of producing protein heterodimers comprises the following steps:
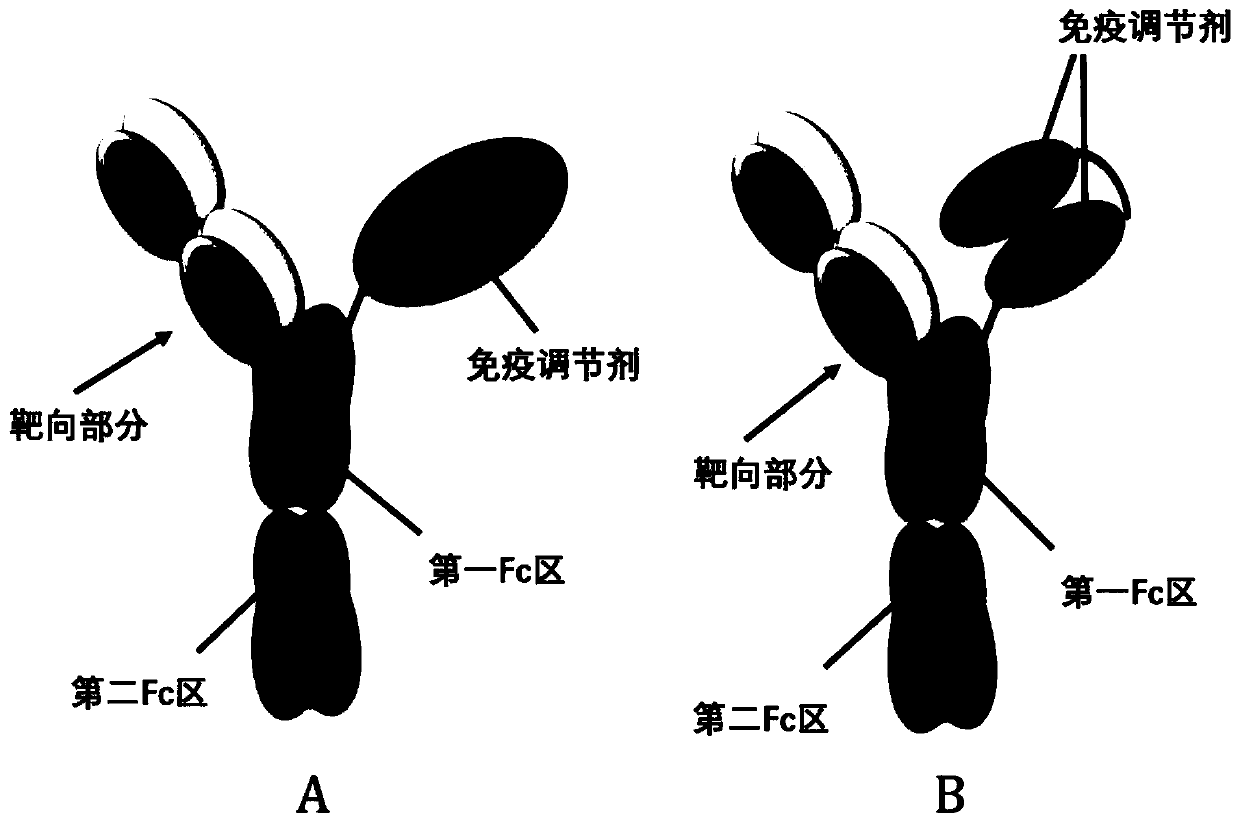
[0408] (1) Provide a first member of a heterodimer, wherein the first member includes a light chain and a heavy chain, and the heavy chain includes a first Fc region, wherein the light chain and the heavy chain are complexed to form a binding specificity for tumor antigens The targeting part;
[0409] (2) Provide a second member of th...
Embodiment approach
[0417] 1. A protein heterodimer comprising a first member and a second member, the second member being different from the first member, wherein: the first member comprises a light chain and a heavy chain, and the heavy The chain includes a first Fc region, and the light chain is complexed with the heavy chain to form a targeting moiety exhibiting binding specificity to tumor antigens; the second member includes a polypeptide that includes a second Fc region Fusion immunomodulator; the first member and the second member associate through the complexation of the first Fc region and the second Fc region to form the heterodimer; the first The Fc region includes a first modification and / or the second Fc region includes a second modification, wherein the first modification and / or the second modification are more effective in promoting all the holes than the hole modification including the node modification and the hole modification. The heterodimerization between the first member and...
Embodiment 1
[0529] Example 1: Modification and preparation of polypeptide
[0530] 1.1 Determine the amino acid modification in the Fc region
[0531] The amino acid residues of the CH3-CH3 domain interface of human IgG1 were determined. Wild-type human IgG1 contains a first heavy chain (chain A) and a second heavy chain (chain B). The respective interface amino acids of chain A and chain B are shown in Table 1 below. The positions of amino acids are determined according to the EU index of KABAT numbering:
[0532]
[0533]
[0534] Table 1. CH3-CH3 interface residues in the Fc region of wild-type human IgG1 antibody (PDB number 1DN2)
[0535] Then, amino acid modifications (such as amino acid substitutions) are made to interface residues to obtain the following modification groups (as shown in Table 2 below, refer to KH knuckle hole modification). In this application, chain A is also referred to as Fc9 or the first Fc region , Chain B is also called Fc6 or the second Fc region:
[0536]
[0537]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com