Strain of pseudomonas aeruginosa producing 5-hydroxymethyl-2 furancarboxylic acid and application of strain of pseudomonas aeruginosa
A technology of Pseudomonas aeruginosa and furanic acid, applied in the biological field, achieves good application prospects, simple operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example illustrates Pseudomonas aeruginosa ( Pseudomonas aeruginosa ) Isolation and Characterization of PC-1
[0034] Pseudomonas aeruginosa ( Pseudomonas aeruginosa ) PC-1.
[0035] (1) After the soil sample was dissolved in deionized water, the supernatant was applied to the screening solid medium for cultivation. The cultivation temperature was 35 °C and the cultivation time was 24-48 h. This method can screen high-concentration 5-hydroxymethylfurfural-resistant microorganisms.
[0036] (2) Pick the strains obtained in step (1) into the enrichment culture medium for enrichment culture at a temperature of 35 °C, and obtain seed liquid after 12-14 hours of culture.
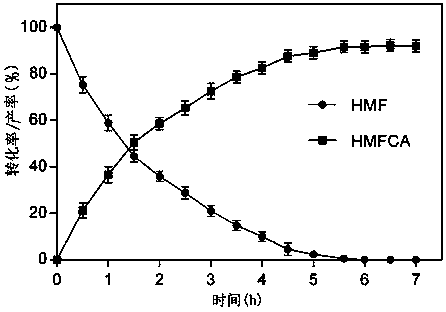
[0037] (3) The seed solution obtained in step (2) was inoculated in a liquid medium containing 100 mM 5-hydroxymethylfurfural at an inoculation volume of 0.5 mg / mL for transformation detection. The conversion temperature was 35 °C, the conversion time was 7 hours, and the rotation speed was 150 ...
Embodiment 2
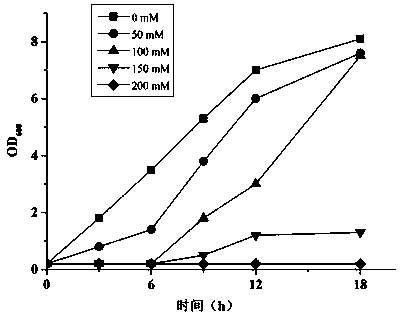
[0041] This example illustrates the effect of 5-hydroxymethylfurfural concentration on Pseudomonas aeruginosa Pseudomonas aeruginosa The effect of growth amount of PC-1 and conversion rate of 5-hydroxymethylfurfural.
[0042]Seed solution was obtained by the method of Example 1. Add different concentrations of 5-hydroxymethylfurfural (50mM, 75mM, 100mM, 150mM, 200mM) to 5 bottles of liquid medium with equal volumes, and take 1 bottle without adding 5-hydroxymethylfurfural as a control. After mixing, each bottle was inoculated with an inoculation amount of 5 mg / mL, and after culturing at 150 rpm and 35 °C for 12 h, the concentration of Pseudomonas aeruginosa PC-1 was measured with a spectrophotometer, and the results were as follows: figure 2 As shown, with the increase of 5-hydroxymethylfurfural concentration, the growth of bacteria was gradually inhibited. When the initial concentration of 5-hydroxymethylfurfural is not higher than 100 mM, the growth of the bacteria is l...
Embodiment 3
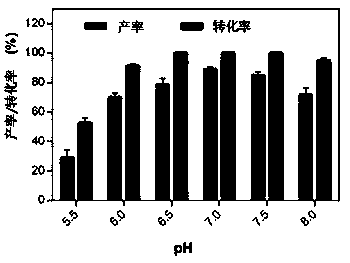
[0044] This example illustrates the impact of different pH on the yield of 5-hydroxymethyl-2 furanic acid and the conversion rate of 5-hydroxymethylfurfural
[0045] After obtaining the seed solution according to the method of Example 1, inoculate the liquid medium containing 100 mM 5-hydroxymethylfurfural at different pH (5.5, 6.0, 6.5, 7.0, 7.5, 8.0) with an inoculation amount of 10 mg / mL After culturing at 150 rpm at 35°C for 6 h, samples were taken for HPLC detection. The result is as image 3 It was shown that when the pH was 7.0, the conversion rate of 5-hydroxymethylfurfural reached 100%, and the yield of 5-hydroxymethyl-2-furancarboxylic acid was the highest, reaching 89.2%, which was the optimal reaction condition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com