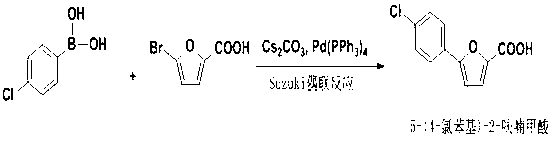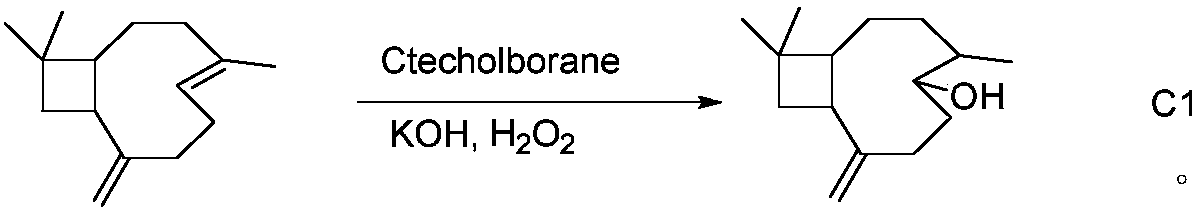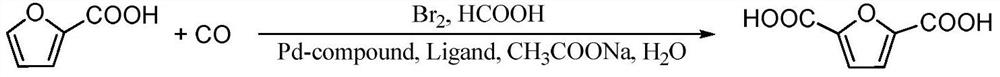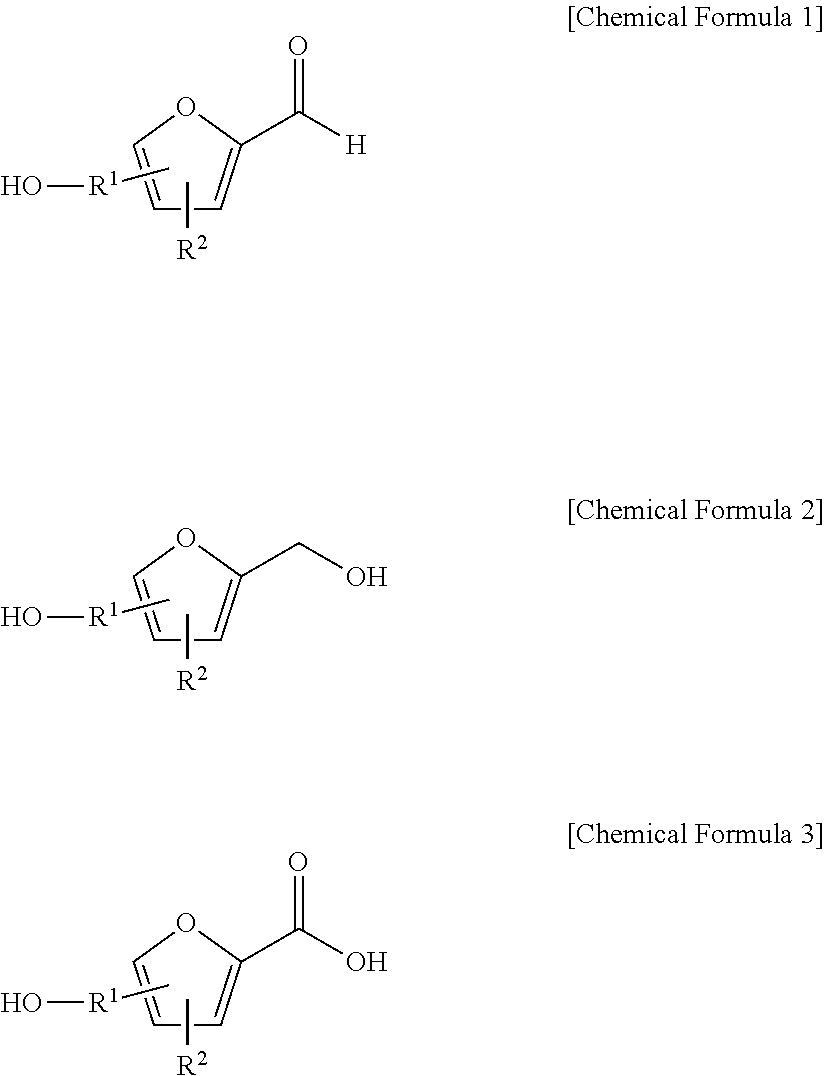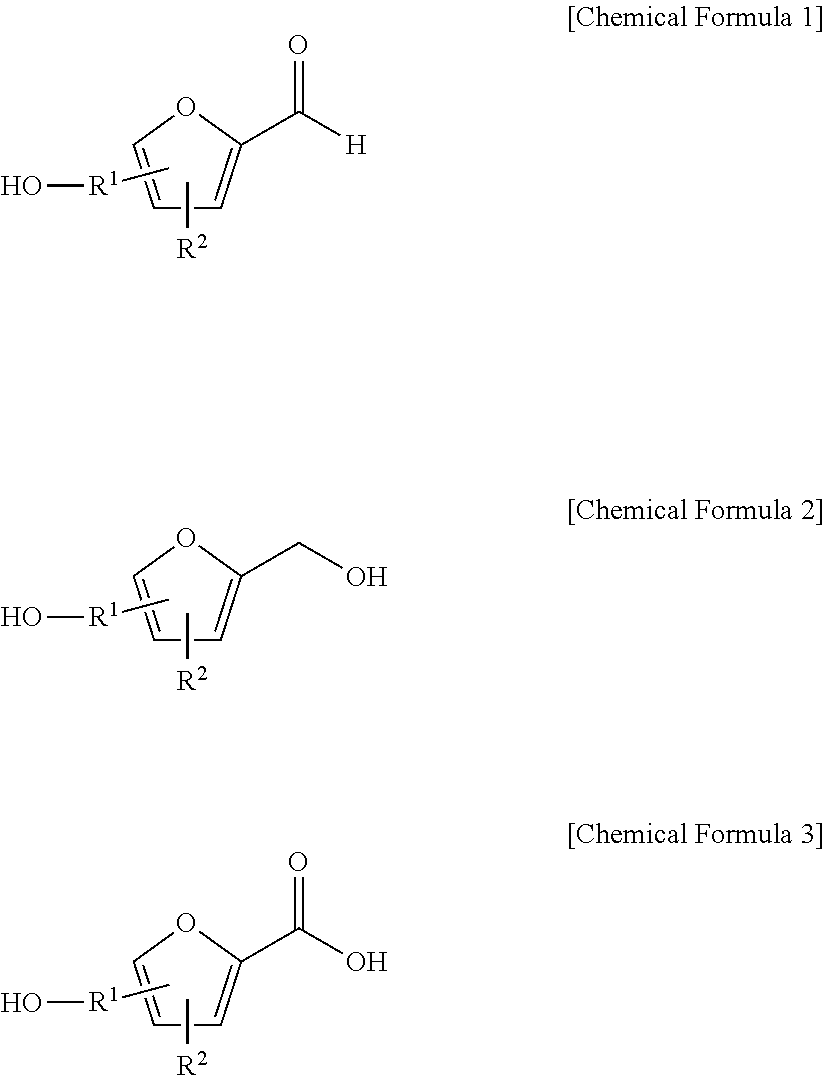Patents
Literature
59 results about "2-furancarboxylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
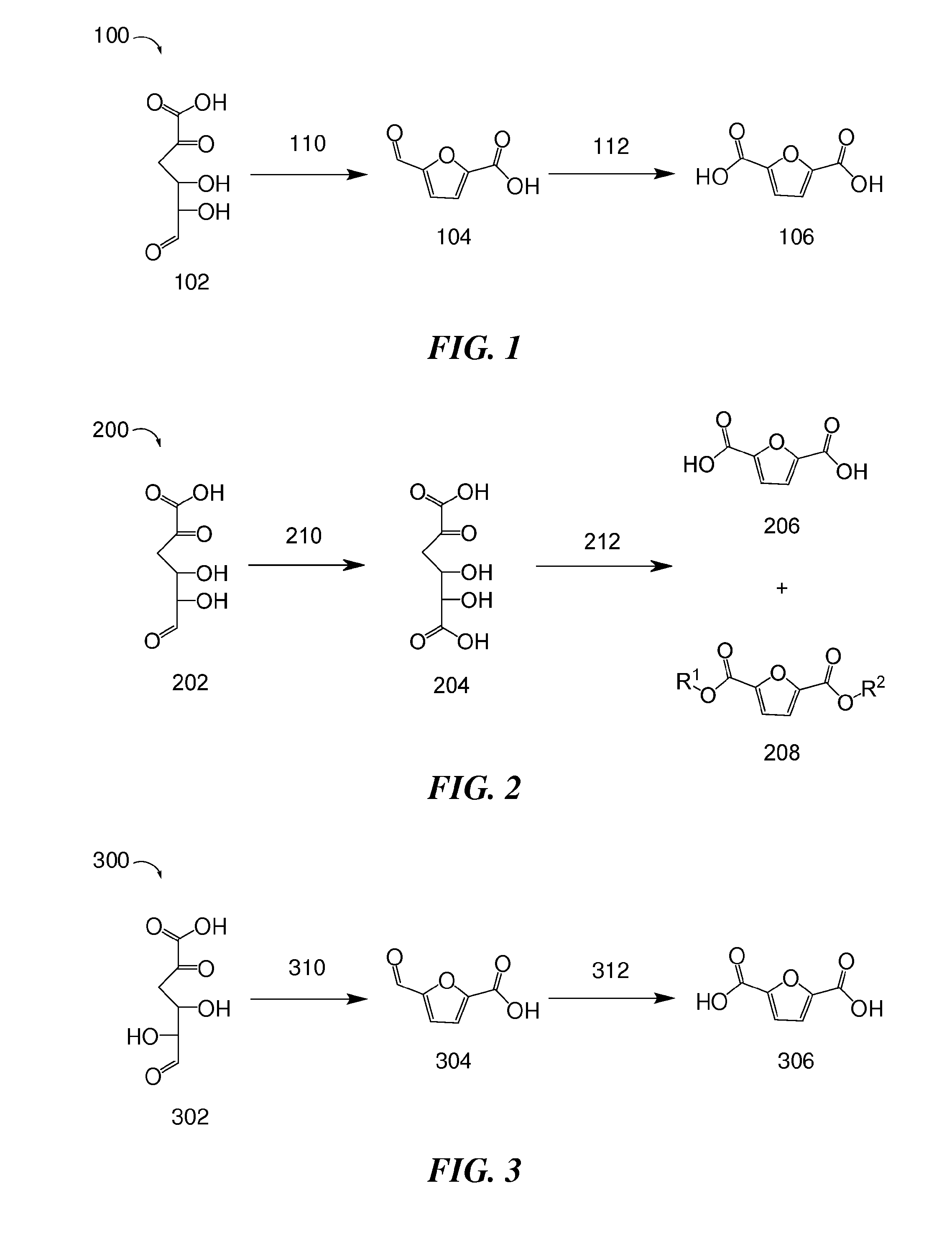
Methods for preparing 2,5-furandicarboxylic acid
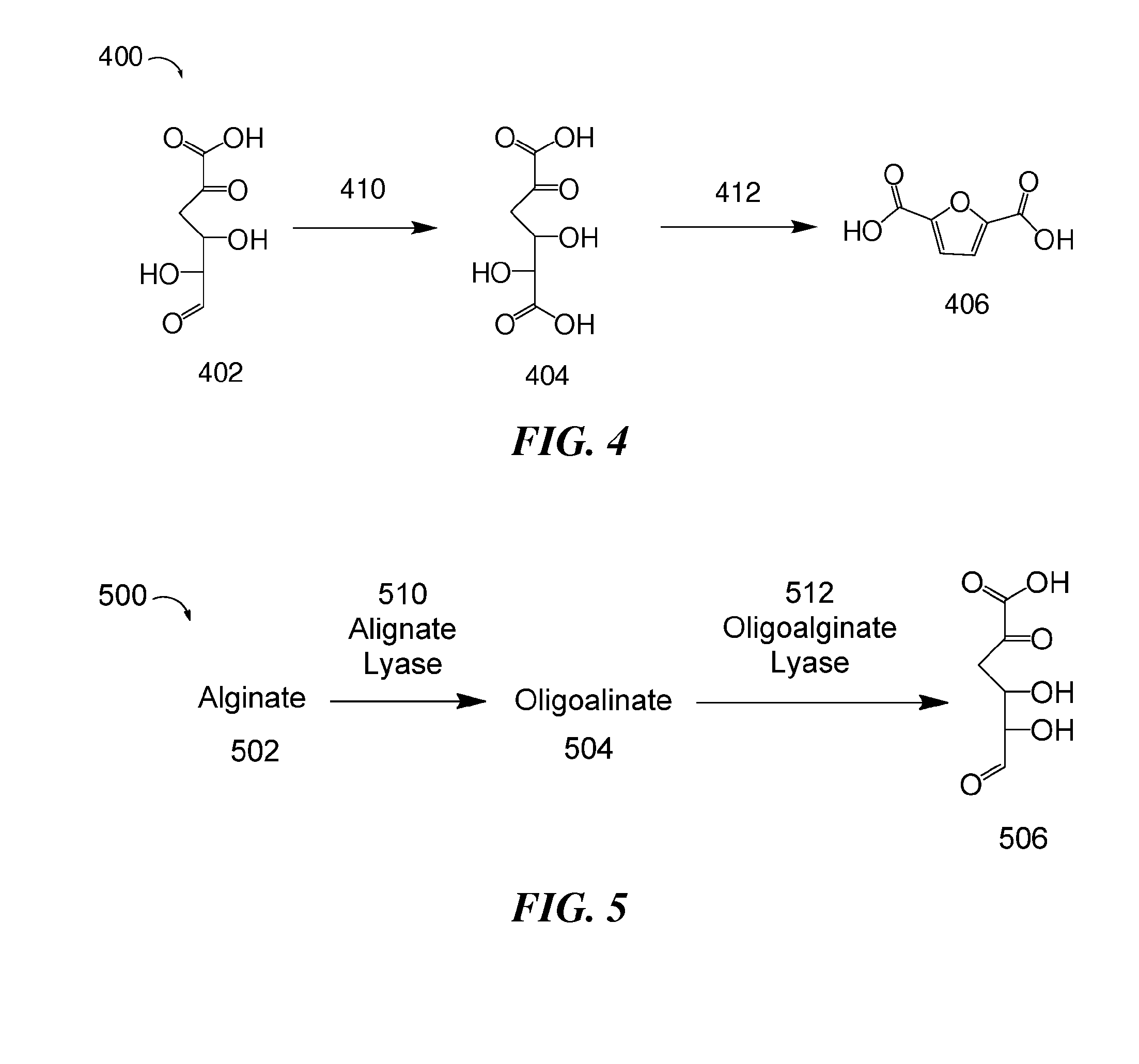
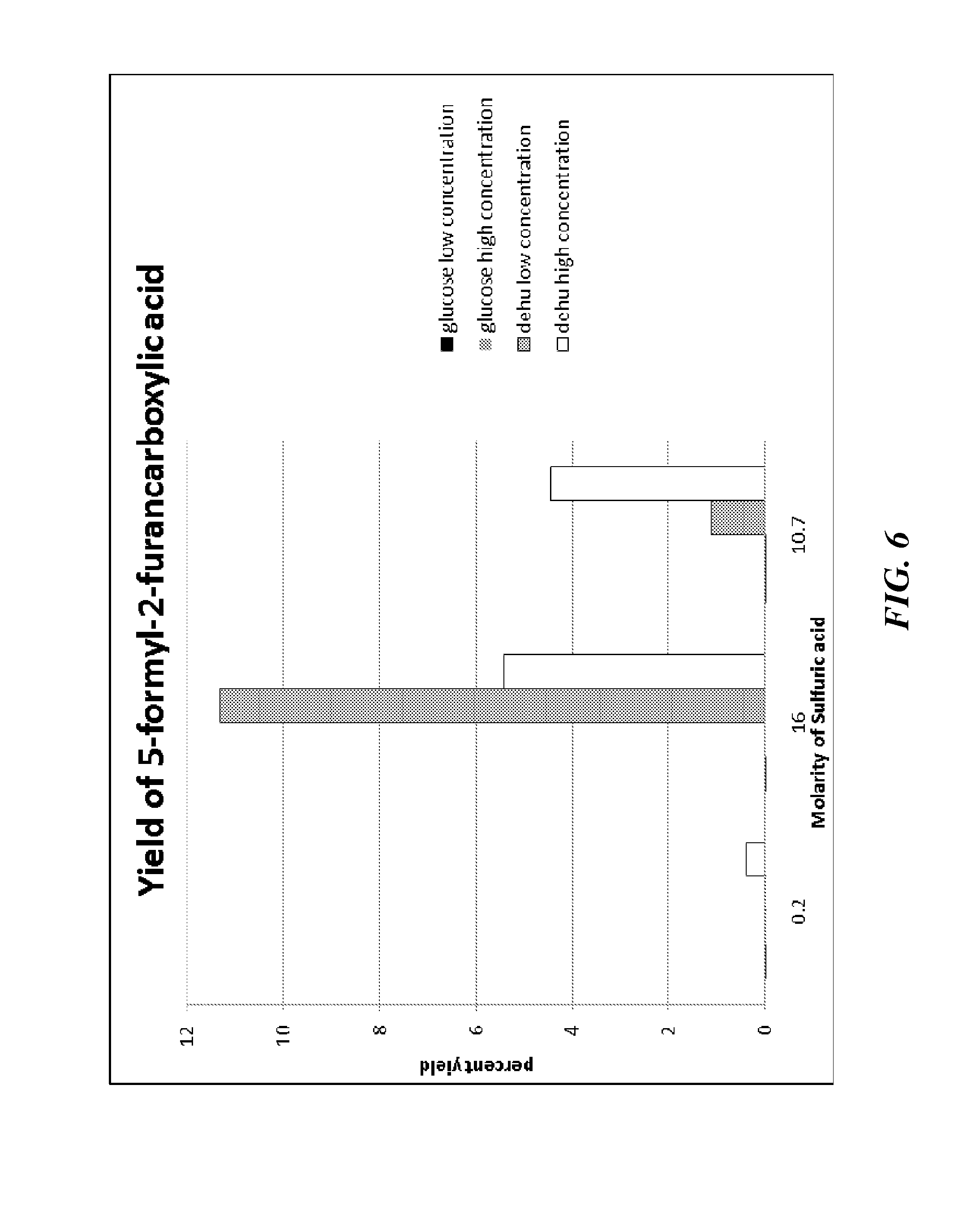
Provided are methods of producing 2,5-furandicarboxylic acid (FDCA) from renewable sources such as seaweed, alginate, oligoalginate, pectin, oligopectin, polygalacturonate, galacturonate, and / or oligogalacturonate. The sugars in the renewable sources can be converted into one or more intermediates such as 4-deoxy-L-erythro-5-hexoseulose uronate (DEHU), 4-deoxy-L-threo-5-hexosulose uronate (DTHU), 5-hydroxymethyl furfural (HMF), 2,5-dihydroxymethyl furan (DHMF), and 5-formyl-2-furancarboxylic acid (FFA), which can be converted into FDCA by dehydration and cyclization to produce 5-formyl-2-furancarboxylic acid (FFA), followed by oxidation to produce FDCA. DEHU or DTHU may also be converted into FDCA by oxidation to produce 2,3-dihydroxy-5-oxohexanedioic acid (DOHA), which then undergoes dehydration and cyclization to produce FDCA.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
2-Furancarboxylic acid hydrazides and pharmaceutical compositions containing the same
InactiveUS20050171196A1High antagonistic activityEasy to disassembleBiocideOrganic chemistryArylHydrogen atom
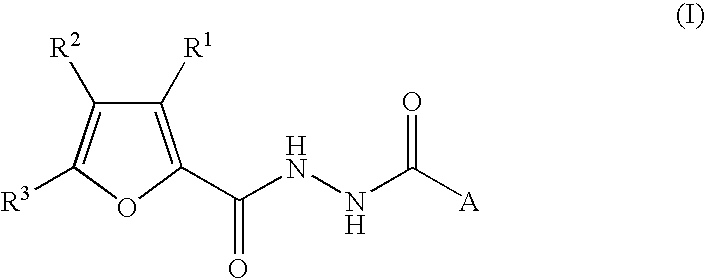
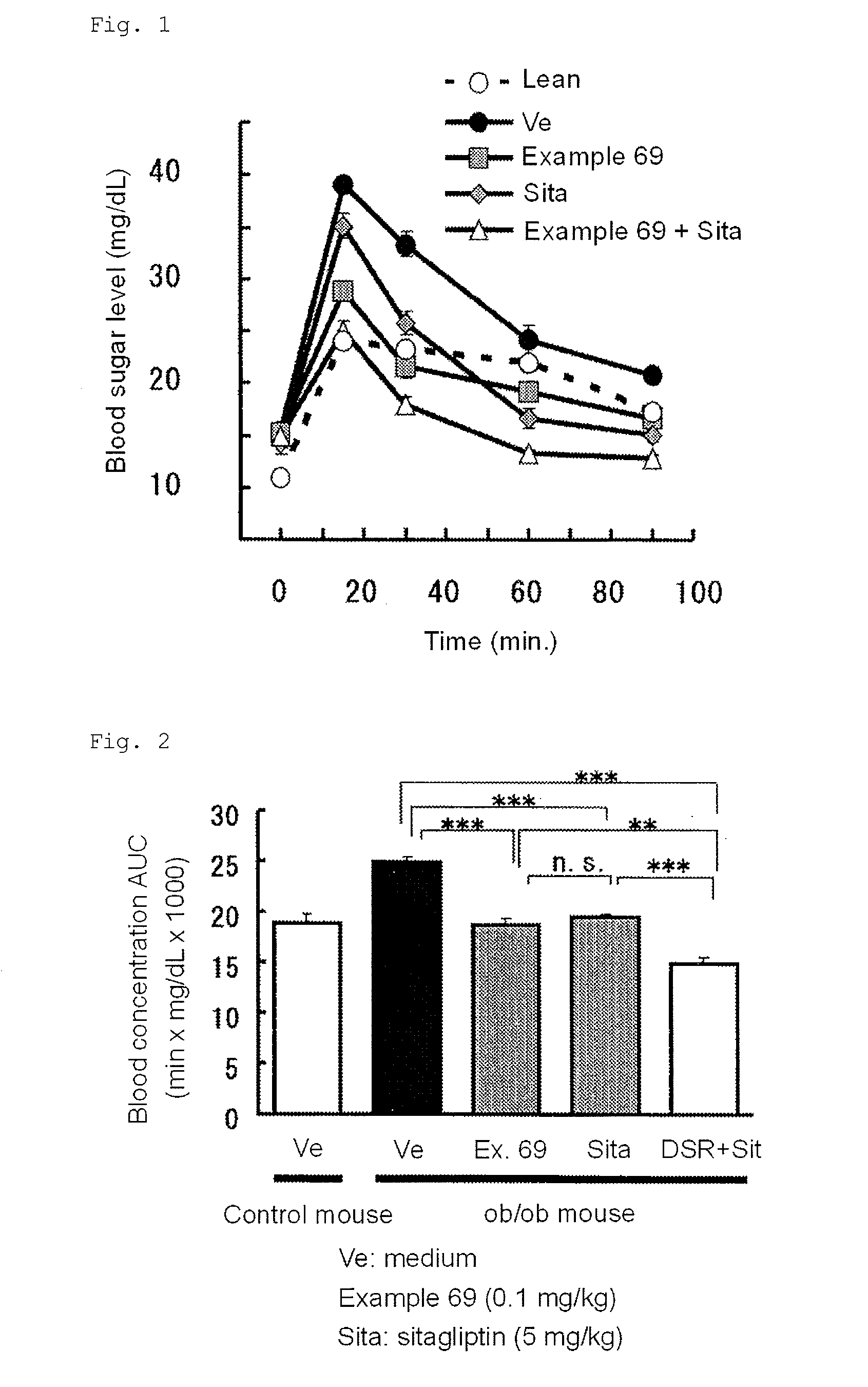
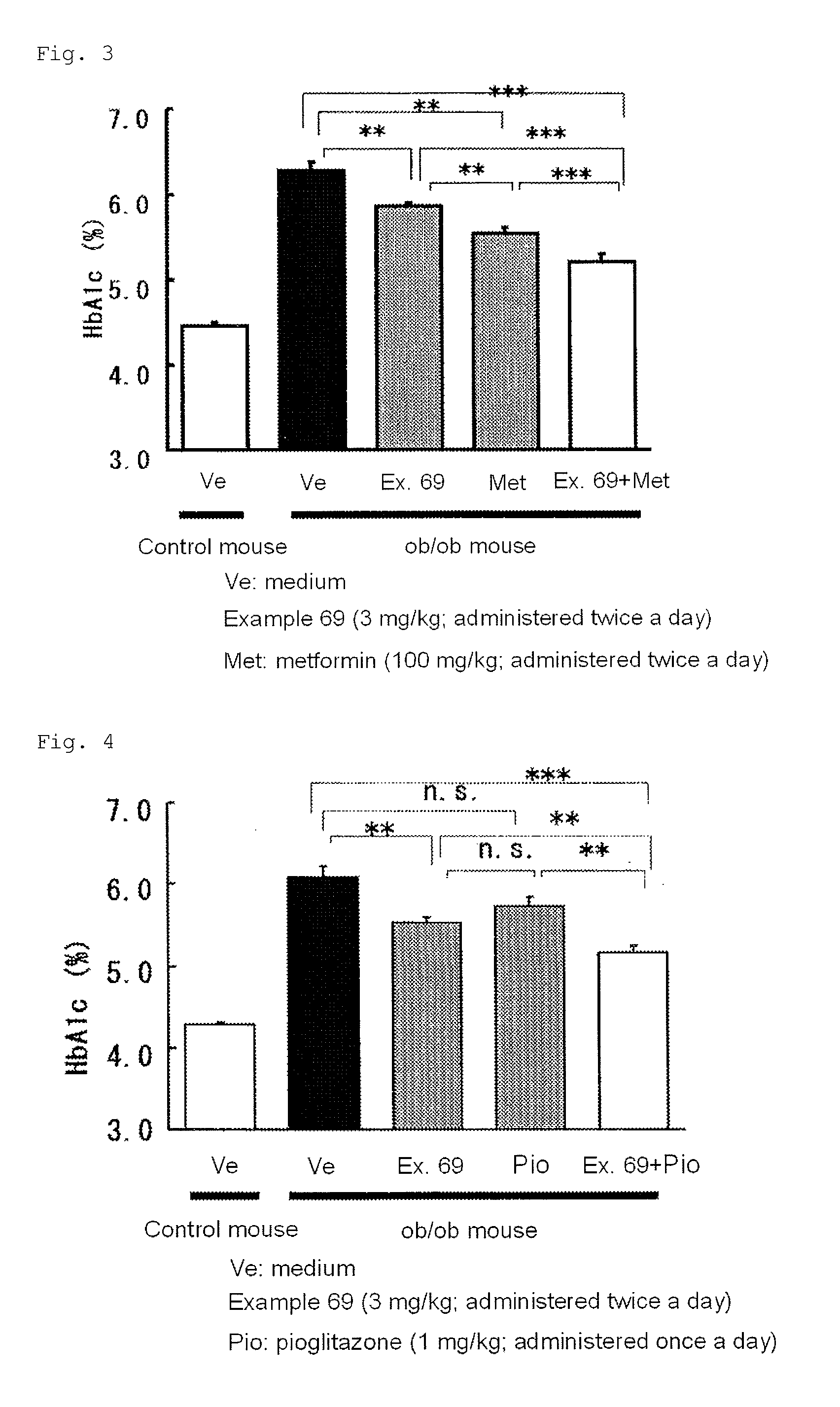
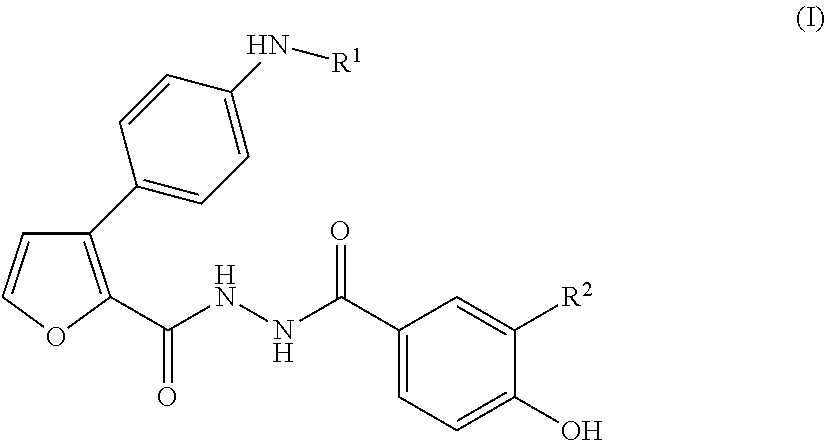
The present invention provides 2-furancarboxylic acid hydrazide compounds represented by General Formula (I) below, and prodrugs, physiologically acceptable salts, hydrates, solvates thereof, methods for producing them and pharmaceutical compositions containing them: wherein A is a group represented by Formula (a) or the like: (wherein either R4 or R5 represents cyano, nitro or the like, and the other represents a hydrogen atom or the like); either R1 or R2 represents a group: -D-(X)m-R6 or the like, and the other represents a group: -E-(Y)n-R7, hydrogen atom, aryl or the like; R3 is a hydrogen atom or the like; D and E independently represent aryl; X and Y independently represent O or the like; R6 and R7 independently represent alkyl, aryl, arylalkyl or the like; and m and n are independently 0 or 1, provided that the aryl is optionally substituted. Such compounds exhibit a potent antagonistic activity on glucagon receptor and are of use as preventive and / or therapeutic agents for symptoms and diseases in which glucagon is involved.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
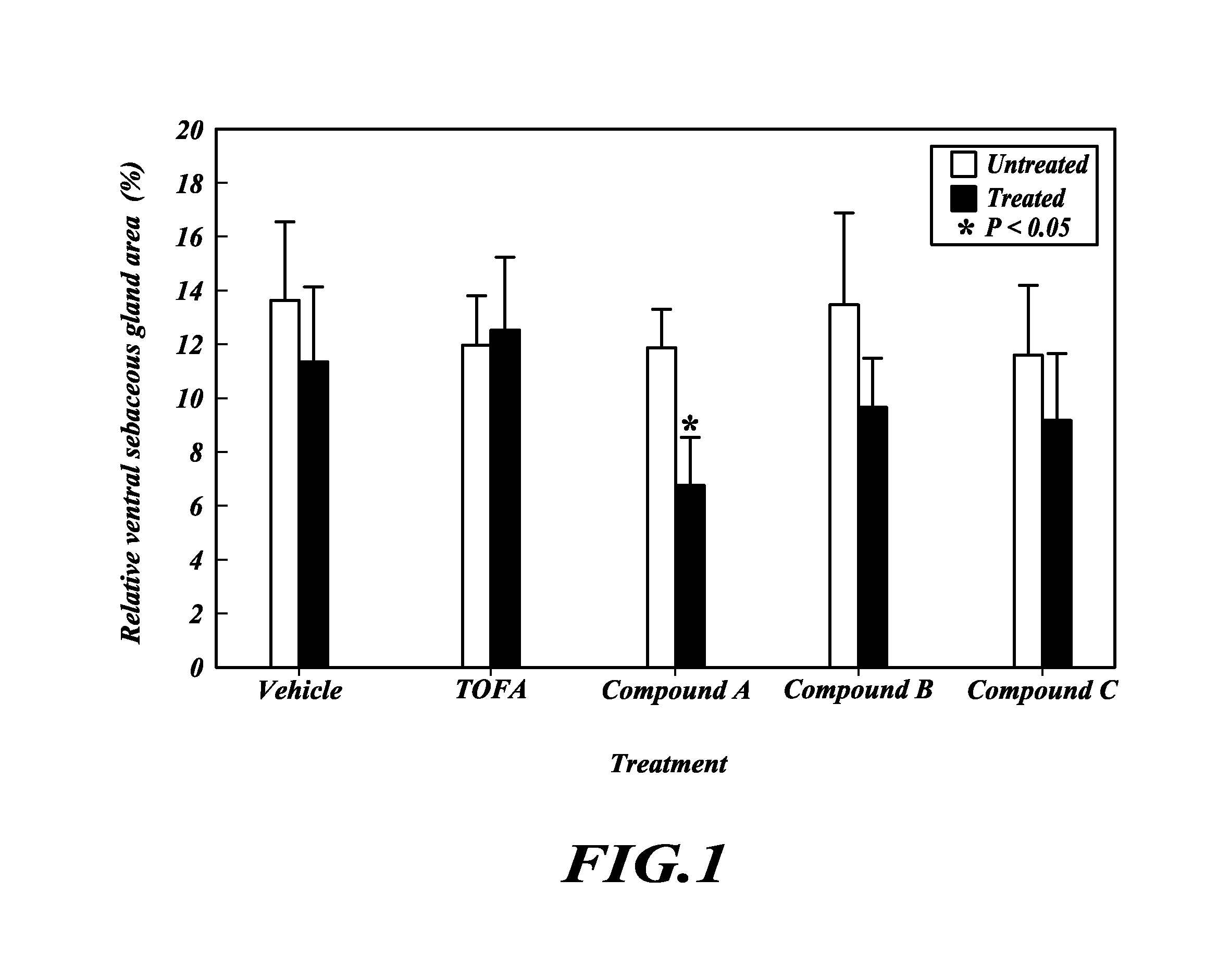
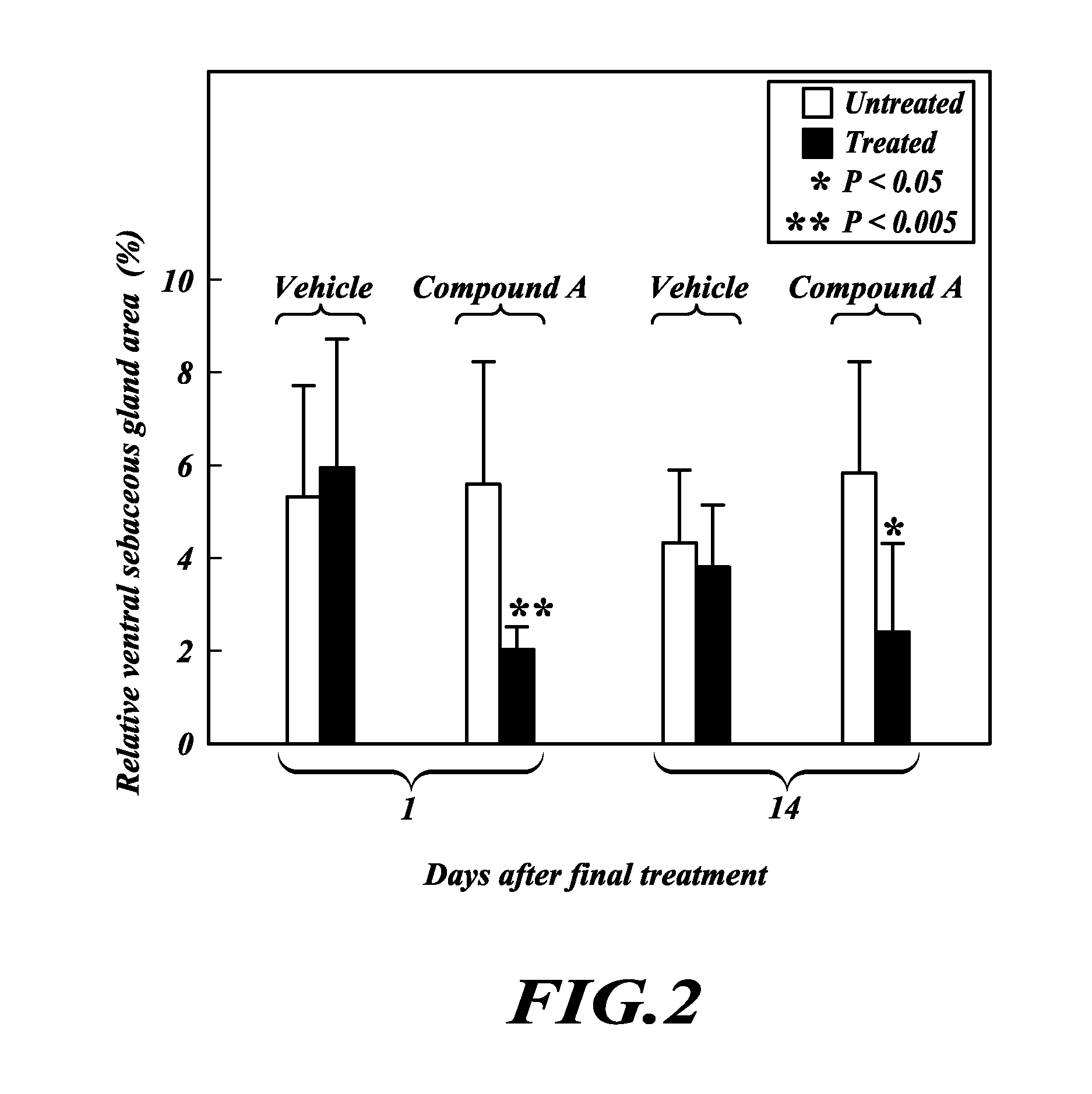
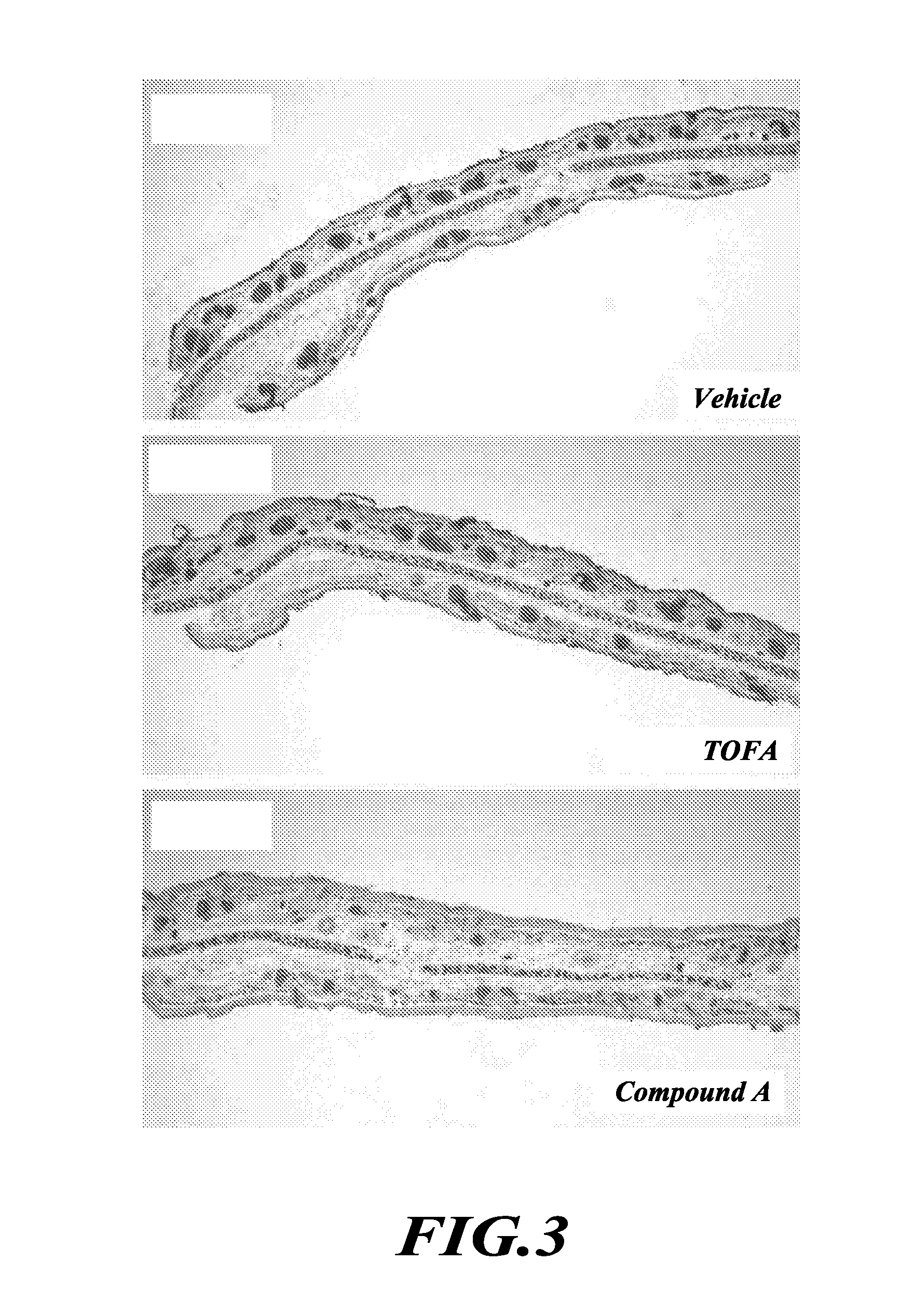
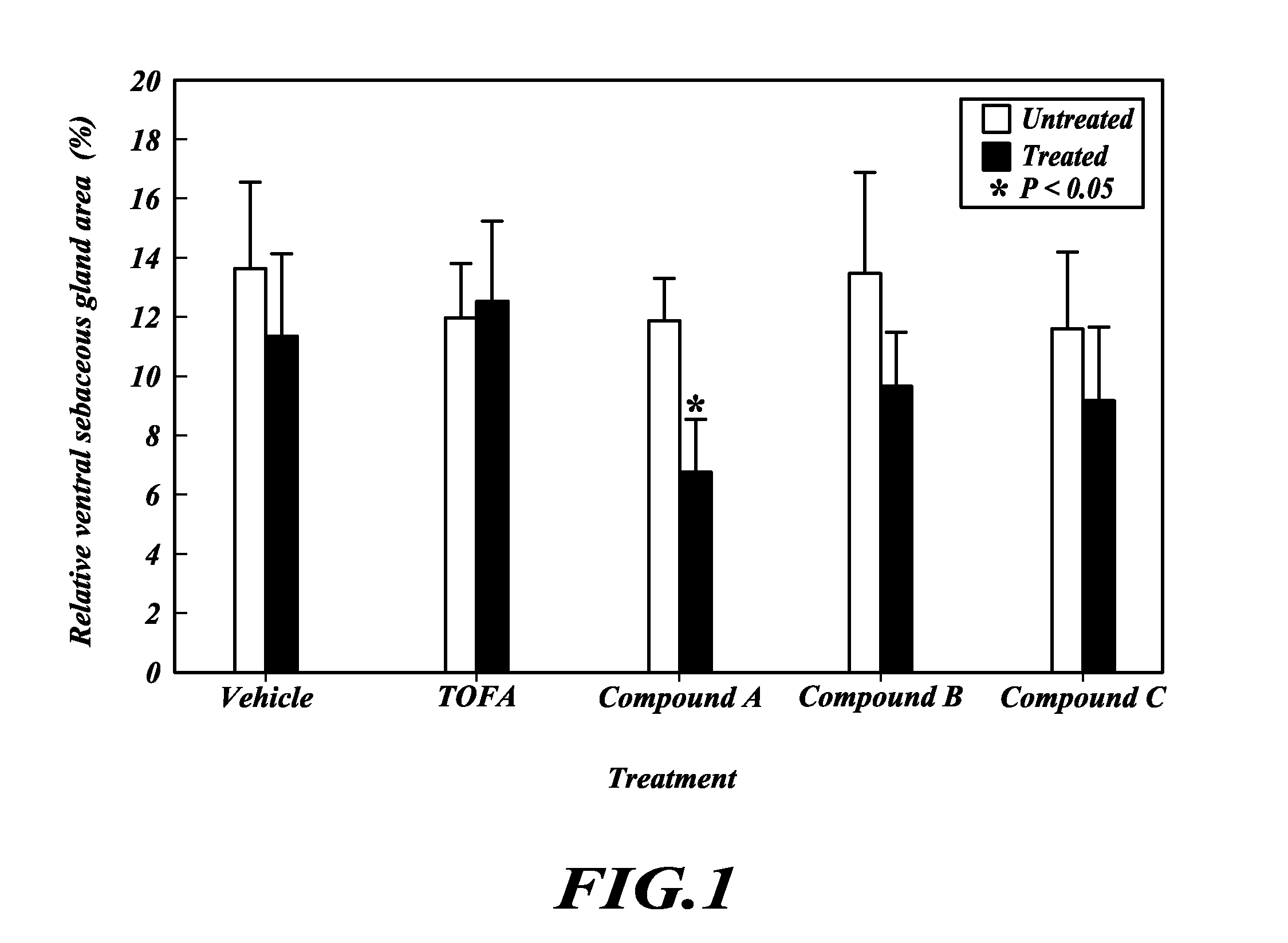
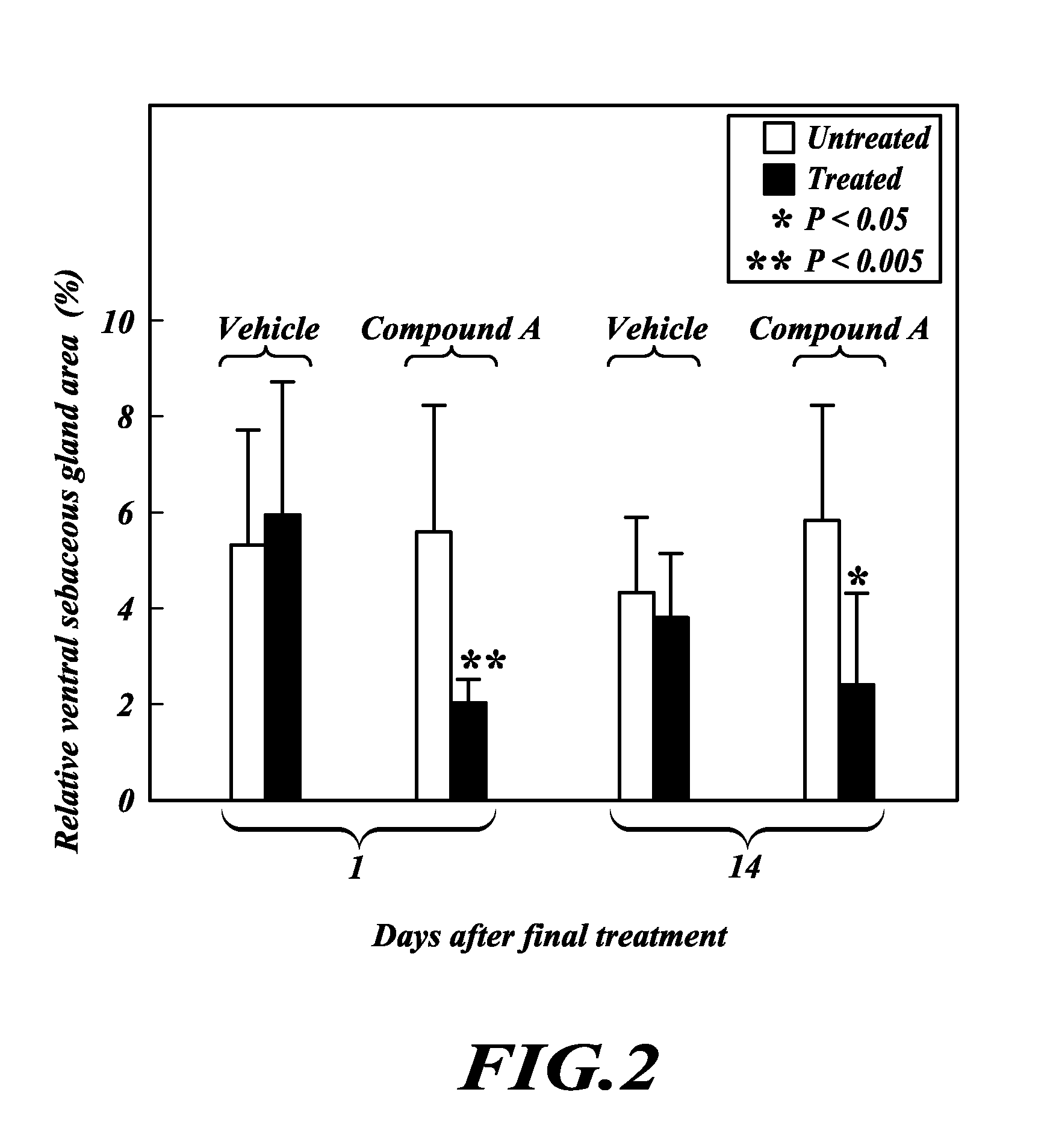
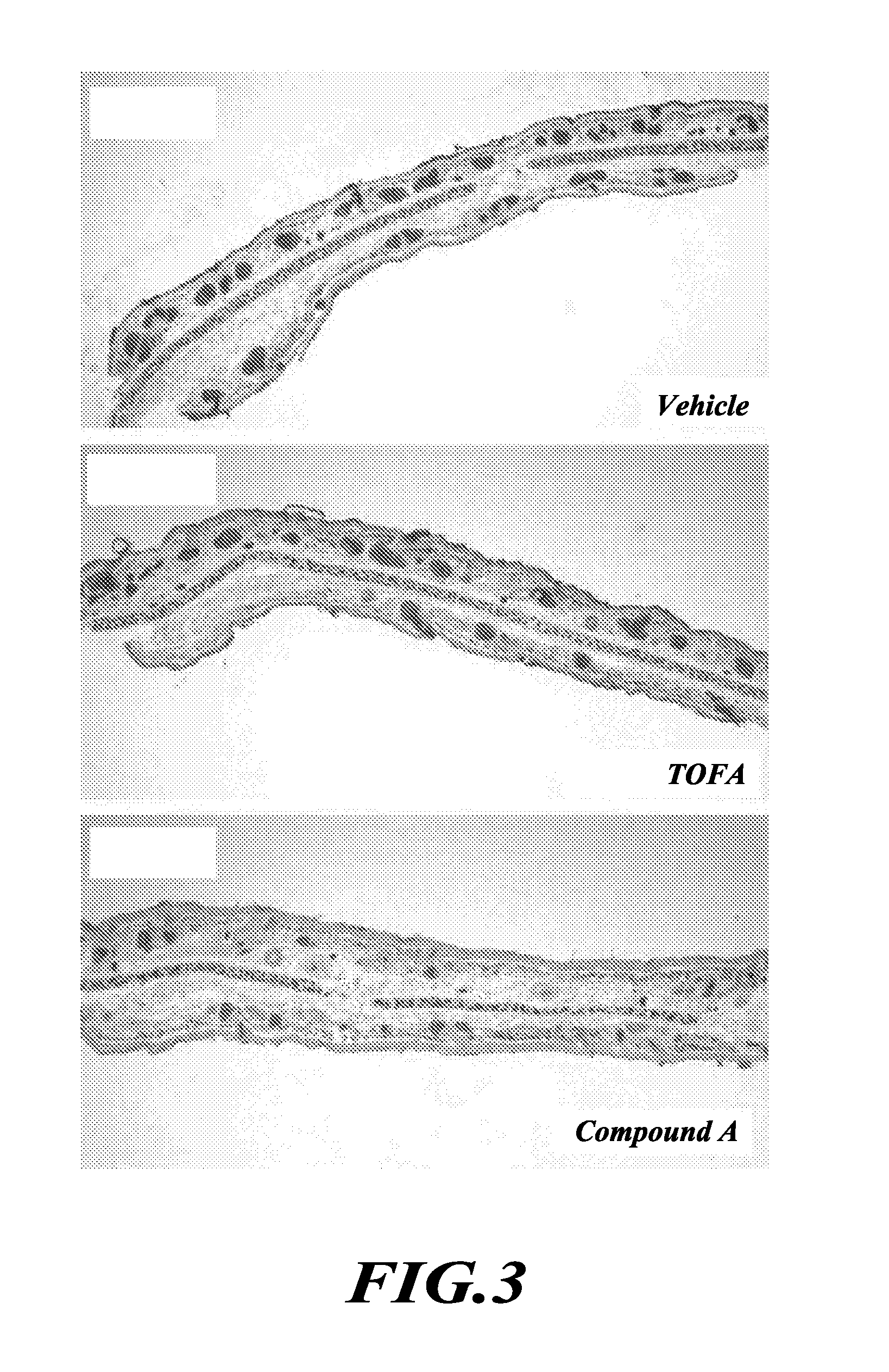
TOFA analogs useful in treating dermatological disorders or conditions
This invention is directed to analogs of 5-(tetradecyloxy)-2-furancarboxylic acid (TOFA) and their use in the treatment of dermatological disorders or conditions characterized by sebaceous gland hyperactivity, such as acne and oily skin, and other dermatological disorders and conditions. This invention is also directed to pharmaceutical compositions comprising analogs of TOFA and a pharmaceutically acceptable excipient for dermatological or oral administration. Formula (I)
Owner:DERMIRA CANADA
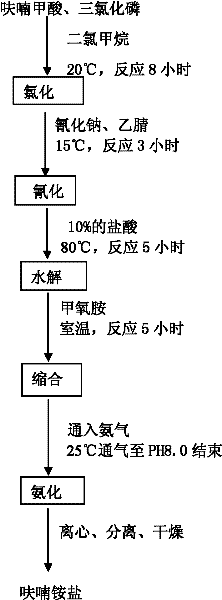
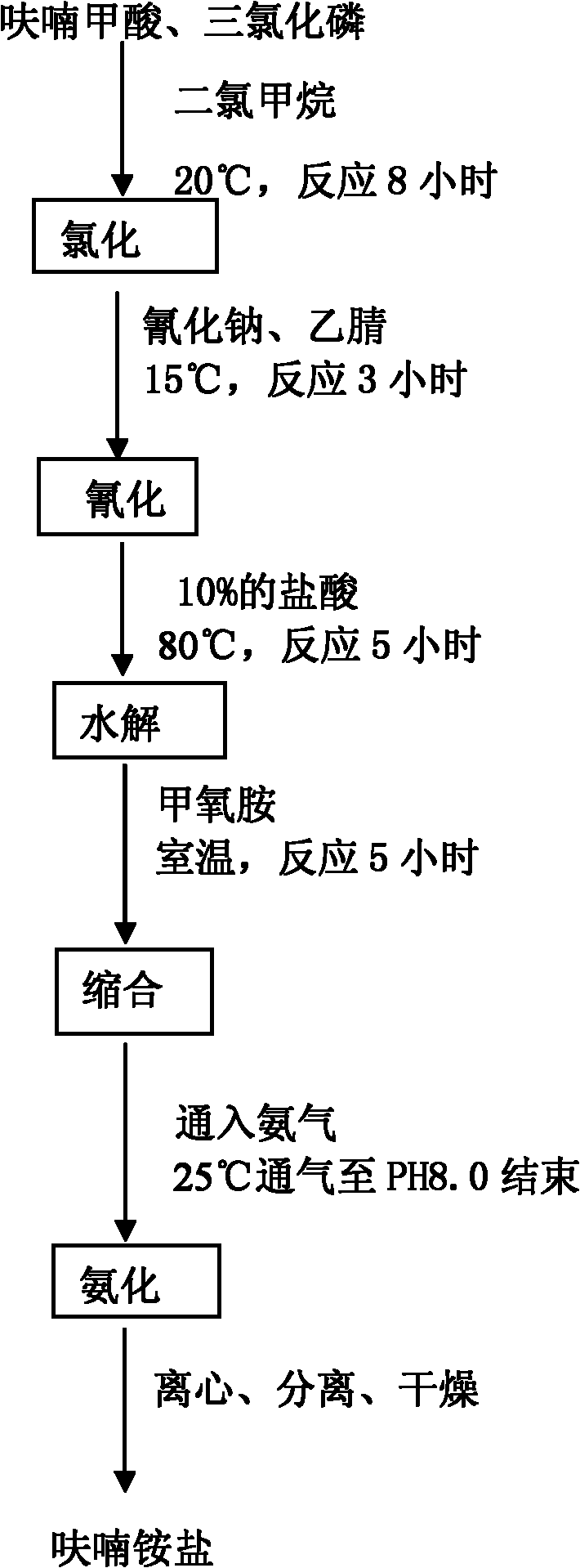
Method for producing furan ammonium salt by using furoic acid
The invention discloses a method for producing a furan ammonium salt by using furoic acid. The method comprises the following steps of: (1) chlorination: putting furoic acid and chlorohydrocarbon solvent into an enameled glass reaction kettle, controlling temperature and time, and obtaining a furoyl chloride product; (2) cyanation: putting the furoyl chloride and sodium cyanide into the reaction kettle, stirring, preserving heat, reducing pressure, and reclaiming the solvent to obtain a furoyl nitrile product; (3) hydrolysis: heating the furoyl nitrile and hydrochloric acid, and obtaining furan ketone acid after the reaction is finished; (4) condensation: adding ethyl acetate extract of the furan ketone acid serving as the hydrolysis product into the reaction kettle, dropping methoxyamine to perform room temperature reaction with the hydrolysis product, and demixing, wherein the oil layer is used for ammoniation reaction; and (5) ammoniation: introducing ammonia gas into the oil layer reactant, controlling the temperature, stirring, finishing gas introduction, centrifuging, and drying to obtain the furan ammonium salt. The method is simple and convenient, is convenient to operate, reduces the production cost, improves the yield, makes full use of raw materials, and is safe and reliable in the production process.
Owner:湖北楚阳科技股份有限公司
3-(4-aminophenyl)-2-furancarboxylic acid derivative and pharmaceutically acceptable salt thereof
InactiveUS20120059012A1Potent glucagon receptor antagonistic activityAvoid symptomsBiocideOrganic chemistryHalogenStereochemistry
Disclosed is a compound represented by Formula (I) or a pharmaceutically acceptable salt thereof:wherein R1 is1: a C3-8 cycloalkyl C1-4 alkyl group,2: a C7-14 aralkyl group, in which the aryl moiety thereof is optionally substituted with the same or different 1 to 3 groups selected from the group consisting of:(a) halogen,(b) C1-4 alkyl, which is optionally substituted with 1 to 3 fluorine atoms,(c) C1-4 alkoxy, which is optionally substituted with 1 to 3 fluorine atoms, and(d) C1-4 alkylcarbonyl, which is optionally substituted with C1-4 alkoxy,3: a five- to ten-membered heteroaryl-C1-4 alkyl group, in which the heteroaryl moiety thereof is optionally substituted with the same or different 1 to 3 groups selected from the group consisting of:(a) halogen, and(b) C1-4 alkyl, or4: a C6-10 aryl C2-6 alkenyl group; andR2 is a cyano group or a nitro group.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Furan glycidyl ether or ester-epoxypropane-CO2 copolymer and preparation thereof
InactiveCN101440158AHigh thermal decomposition temperatureMaintain full biodegradabilityFuranCopolymer
The invention provides a copolymer of furan-type glycidol ether or furan-type glycidyl ester and propylene oxide and CO2, and a preparation method thereof. The copolymer is prepared by polymerizing the furan-type glycidol ether or furan-type glycidyl ester with the propylene oxide and carbon dioxide under the action of a rare-earth three-way catalyst, wherein the furan-type glycidol ether is furan methyl glycidol ether, 5-methylfuran methyl glycidol ether or tetrahydrofuran methyl glycidol ether; the furan-type glycidyl ester is furan formic-acid glycidyl ester, 5-methylfuran formic-acid glycidyl ester or tetrahydrofuran formic-acid glycidyl ester; and the furan-type glycidol ether or furan-type glycidyl ester can be prepared from furan alcohol or furan acid which is a renewable resource. The highest yield of the terpolymer is up to 4.298*10<3> grams of copolymer per mol of Zn. The initial thermal decomposition temperature of the terpolymer is 256 DEG C, which is 69 DEG C higher than the initial thermal decomposition temperature (187 DEG C) of PPC.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for producing furandicarboxylic acid and derivatives thereof from furfural
The invention discloses a method for producing furandicarboxylic acid and derivatives thereof from furfural. The method comprises the following steps: furfural is reduced to 2-methylfuran under the hydrogen condition; acetylation reaction is carried out on 2-methylfuran to obtain 5-methyl-2-acetylfuran; 5-methyl-2-acety furan reacts with ester to obtain methyl 5-methyl-2-furanformate, methyl 5-methyl-2-furanformate is oxidized into monomethyl 2,5-furandicarboxylate under the oxygen condition, and monomethyl 2,5-furandicarboxylate is hydrolyzed into monomethyl 2,5-furandicarboxylate or furtheresterified with methyl alcohol to generate dimethyl 2,5-furandicarboxylate. The cheap five-carbon furan compound furfural is used as a raw material, and the 2 5-furandicarboxylic acid and the derivatives thereof are prepared by a strategy of increasing a carbon chain, so that the cost of the raw material is greatly reduced. The product provided by the invention has high purity and can be directlyused as a polymerization monomer of PET polyester.
Owner:EAST CHINA NORMAL UNIVERSITY
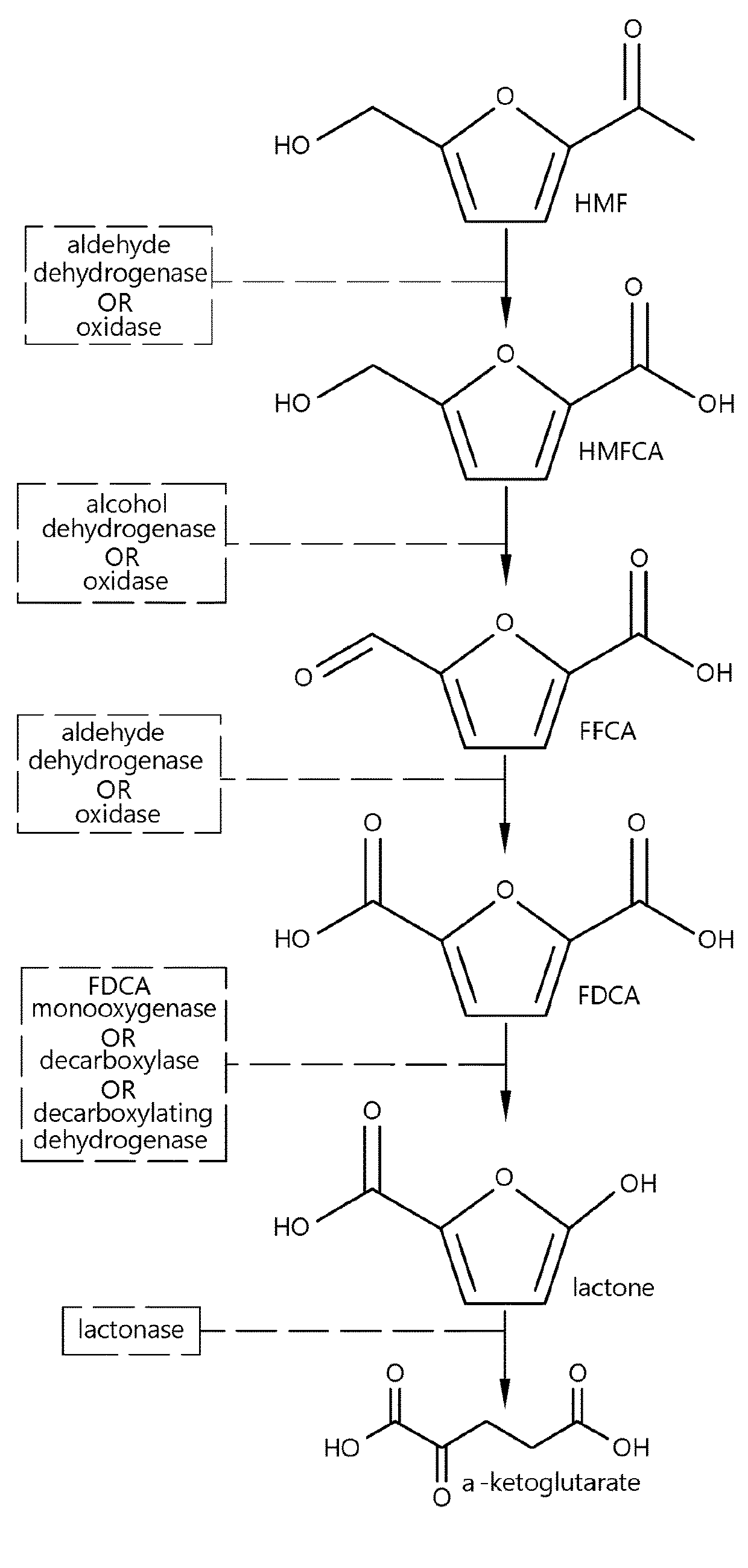
5-hydroxymethylfurfural oxidase gene HMFO and codase thereof as well as application
ActiveCN108118064AHigh activityCatalytic reaction time is shortMicroorganism based processesNucleic acid vectorFuranPseudomonas nitroreducens
The invention provides pseudomonas nitroreducens 5-hydroxymethylfurfural oxidase and a preparation method thereof. Particularly, the DNA sequence of 5-hydroxymethylfurfural oxidase is cloned to plasmid, and recombinant plasmid is integrated into host bacteria to obtain a gene engineering strain capable of heterologous expression of the oxidase. The prepared 5-hydroxymethylfurfural oxidase subjected to heterologous expression of the strain can oxidize the 5-hydroxymethylfurfural oxidase and convert the 5-hydroxymethylfurfural oxidase into 2,5-furandicarboxylic acid, furan-2,5-dicarbaldehyde and5-formyl-2-furancarboxylic acid. The invention provides a technological base for preparing furan bulk chemicals by oxidizing 5-hydroxymethylfurfural through bioanalysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
TOFA analogs useful in treating dermatological disorders or conditions
InactiveUS20120208807A1Inhibitory activityReducing cytokine secretionBiocideOrganic chemistryFuranDERMATOLOGY/SKIN
This invention is directed to analogs of 5-(tetradecyloxy)-2-furancarboxylic acid (TOFA) and their use in the treatment of dermatological disorders or conditions characterized by sebaceous gland hyperactivity, such as acne and oily skin, and other dermatological disorders and conditions. This invention is also directed to pharmaceutical compositions comprising analogs of TOFA and a pharmaceutically acceptable excipient for dermatological or oral administration. Formula (I)
Owner:DERMIRA CANADA
Bio-based benzoxazine resin containing furanamide structure and preparation method of bio-based benzoxazine resin
The invention discloses bio-based benzoxazine resin containing a furanamide structure and a preparation method of the bio-based benzoxazine resin. Biomass such as furancarboxylic acid and derivativesthereof is used as a raw material, furan groups are bonded into a phenol source structure of the benzoxazine resin through amido bonds by means of a condensation reaction, Mannich reaction is carriedout with monobasic or polybasic primary amine compounds such as furanmethylamine to prepare the semi-bio-based or full-bio-based benzoxazine resin containing the furanamide structure. The bio-based benzoxazine resin containing the furanamide structure has high crosslinking degree, thermal polymerization characteristic and high thermal stability, achieves green synthesis and high performance of thebenzoxazine resin, expands the application range of the benzoxazine resin, and can be applied to the fields of electrical insulation, aerospace ablation-resistant materials, aviation structure materials, electronic packaging materials, flame-retardant materials and the like.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING +1
Cherry Maotai-flavor Baijiu and preparation method thereof
InactiveCN107058021AWith fragranceGood sauceAlcoholic beverage preparationRhizopus oryzaeTriticum turgidum
The invention discloses cherry Maotai-flavor Baijiu and a preparation method thereof. The preparation method comprises the following steps: preparing materials: based on mass percent, weighing 85 percent to 90 percent of sorghum and 10 percent to 15 percent of wheat as raw materials; based on mass percent, weighing 18 percent to 22 percent of rhizopus oryzae bran koji, 45 percent to 50 percent of aroma-producing yeast and 30 percent to 35 percent of lactic acid bacteria bran koji as auxiliary materials; weighing cherries which account for 3 percent to 7 percent of the mass of the sorghum for later use; after moistening the materials, steaming the materials under the pressure of 0.4MPa to 0.5MPa; after spreading and cooling the cooked materials, uniformly mixing the materials with a cherry mixture; after uniformly mixing with the auxiliary materials, accumulating and fermenting; putting the materials into a cellar and distilling to obtain the cherry Maotai-flavor Baijiu. The Baijiu prepared by the process has a cherry flavor, has a good Maotai flavor and a full wine body, is mellow and soft, and has an abundant taste. The Baijiu prepared by the preparation method contains ligustrazine, chitin, maltol, borneol, 2-furancarboxylic acid, ferulic acid and the like, and has a health-care effect. According to the cherry Maotai-flavor Baijiu and the preparation method thereof, the reinvestment is reduced, so that a whole production process is simple to operate, the cost is low, the period is short, and popularization is easy .
Owner:安徽徽酒酒业有限公司
Strain of pseudomonas aeruginosa producing 5-hydroxymethyl-2 furancarboxylic acid and application of strain of pseudomonas aeruginosa
ActiveCN110724654AToleratedEasy to operateBacteriaMicroorganism based processesBiotechnologyFuraldehyde
The present invention discloses a strain of pseudomonas aeruginosa producing 5-hydroxymethyl-2 furancarboxylic acid and an application of the strain of the pseudomonas aeruginosa, and belongs to the technical field of biology. The disclosed pseudomonas aeruginosa PC-1 has a preservation number of CCTCC NO:M2019537. The strain is used to transform 5-hydroxymethylfurfural into 5-hydroxymethyl-2 furancarboxylic acid and has advantages of short reaction time, mild conditions, no pollution to the environment, and high product selectivity. At the same time, the present invention also provides an application of the strain in preparing the 5-hydroxymethylfurfural, product yield is high, and the strain has a broad application prospect.
Owner:NANJING POLYTECHNIC INSITUTE +2
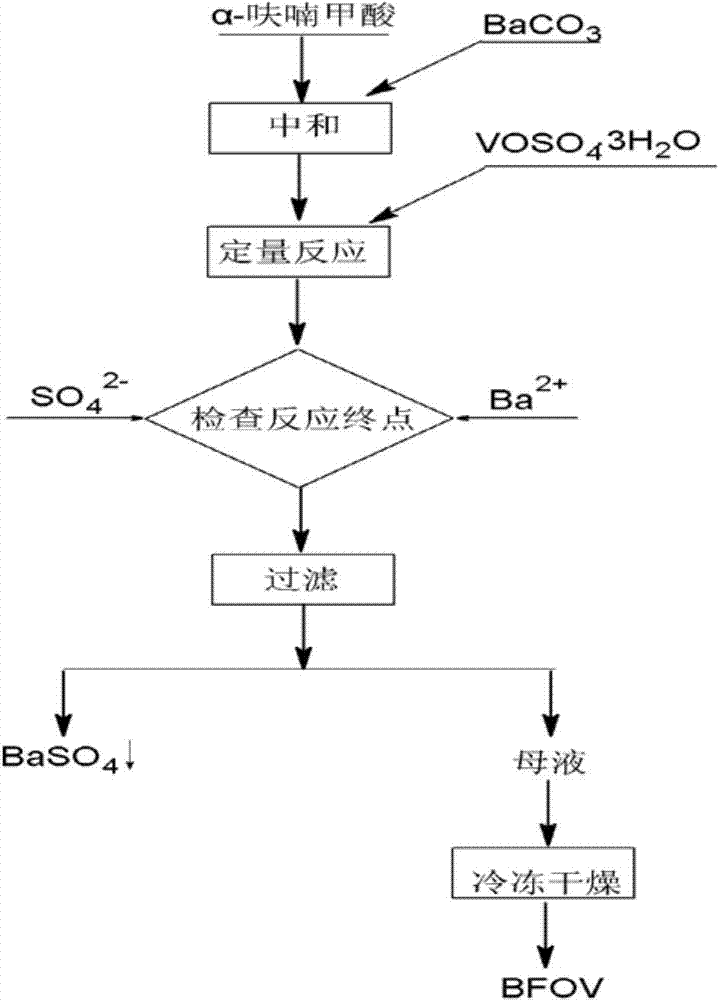
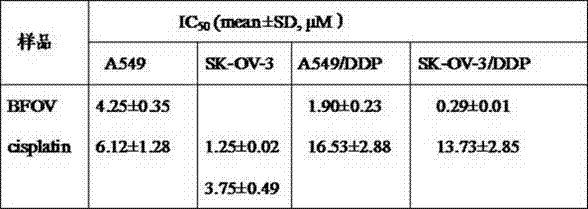
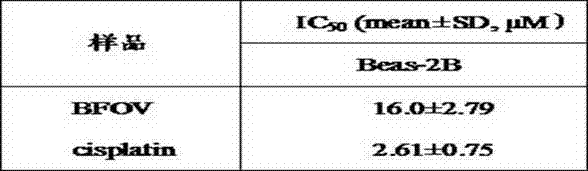
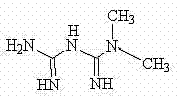
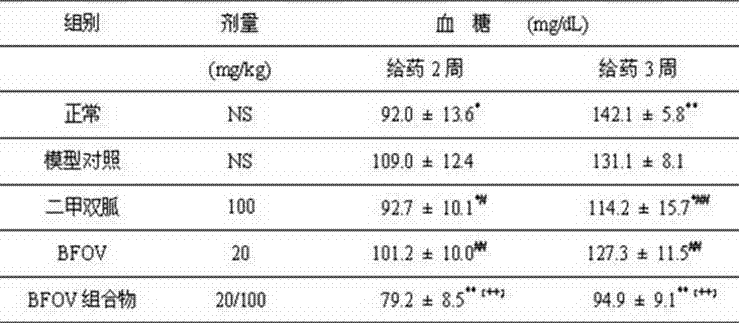
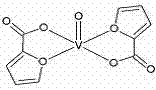
Bis(alpha-furancarboxylato)oxovanadium composition for treatment of diabetes
InactiveCN103316017AImprove stabilityReduced stabilityOrganic active ingredientsMetabolism disorderPhysiologyPharmaceutical Aids
The invention discloses a bis(alpha-furancarboxylato)oxovanadium composition for treatment of diabetes, and belongs to the field of pharmaceutics. The composition of the invention is prepared from bis(alpha-furancarboxylato)oxovanadium and melbine at a ratio of 1:5 to 50, and appropriate accessories are added to produce pills, powders and capsules. Recommended dosage for human is 10mg: (50 to 500mg) per day, and preferable dosage is 10mg:200mg or 10mg:500mg. The results of animal experiment show that: the composition is capable of decreasing blood glucose levels of alloxan-induced diabetic mice, streptozotocin-induced diabetic mice and high fat feed-induced insulin-resistant mice greatly, improving lipid metabolism disorders of insulin-resistant mice, and increasing insulin sensitivity.
Owner:KUNMING MEDICAL UNIVERSITY +2
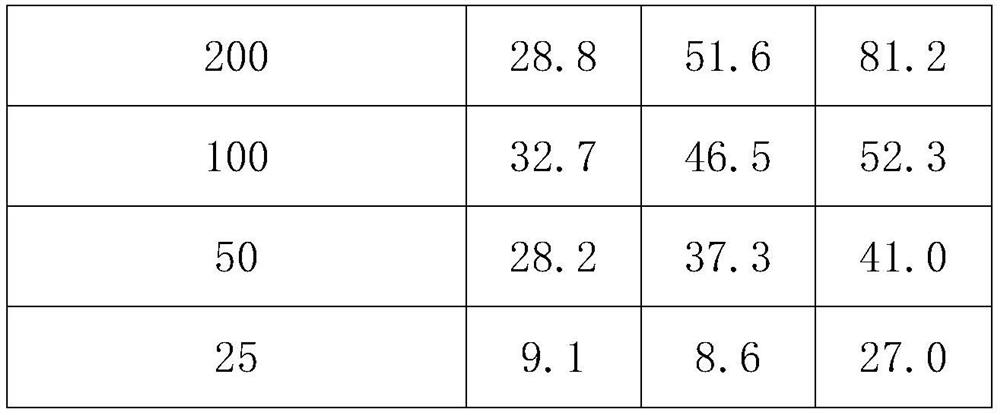
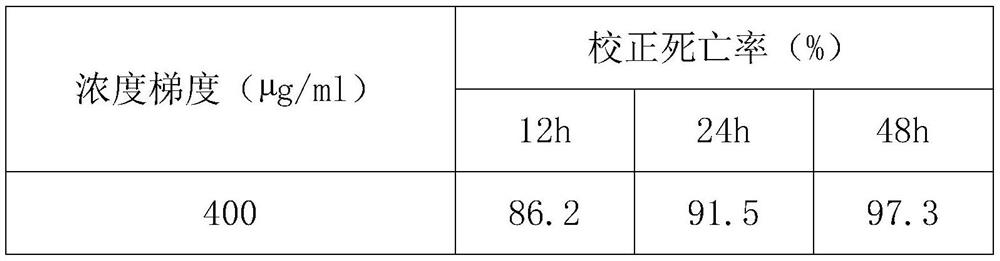
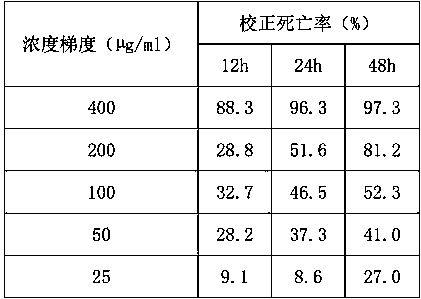
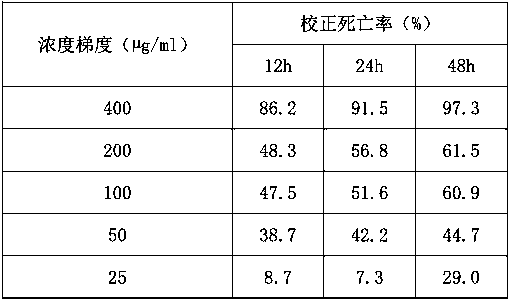
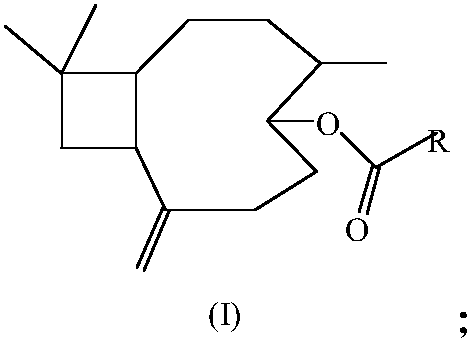
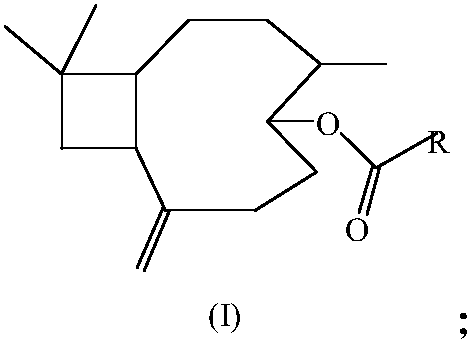
17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof
The invention relates to 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate as well as an extraction method and medicine application thereof, and belongs to a new compound and medicine application thereof. Ginseng stem and leaves and artemisias tolonifera are taken as raw materials and are subjected to pulverization, high temperature high pressure treatment, drying, chloroform extraction, silica-gel column chromatography and recrystallization, and then the 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate is obtained. The 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate is applied to preparation of medicines for treating the hypertension.
Owner:JILIN AGRICULTURAL UNIV
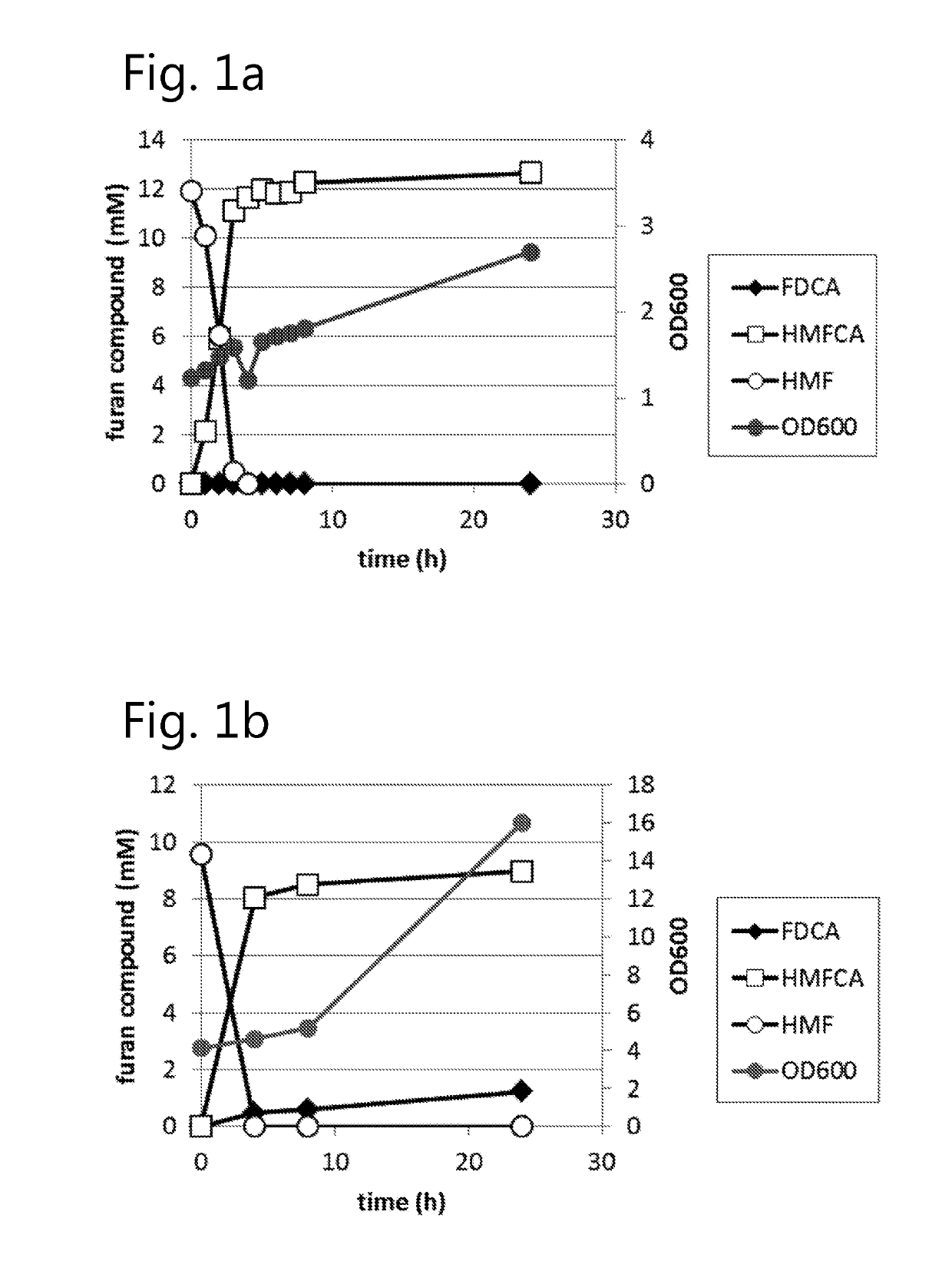
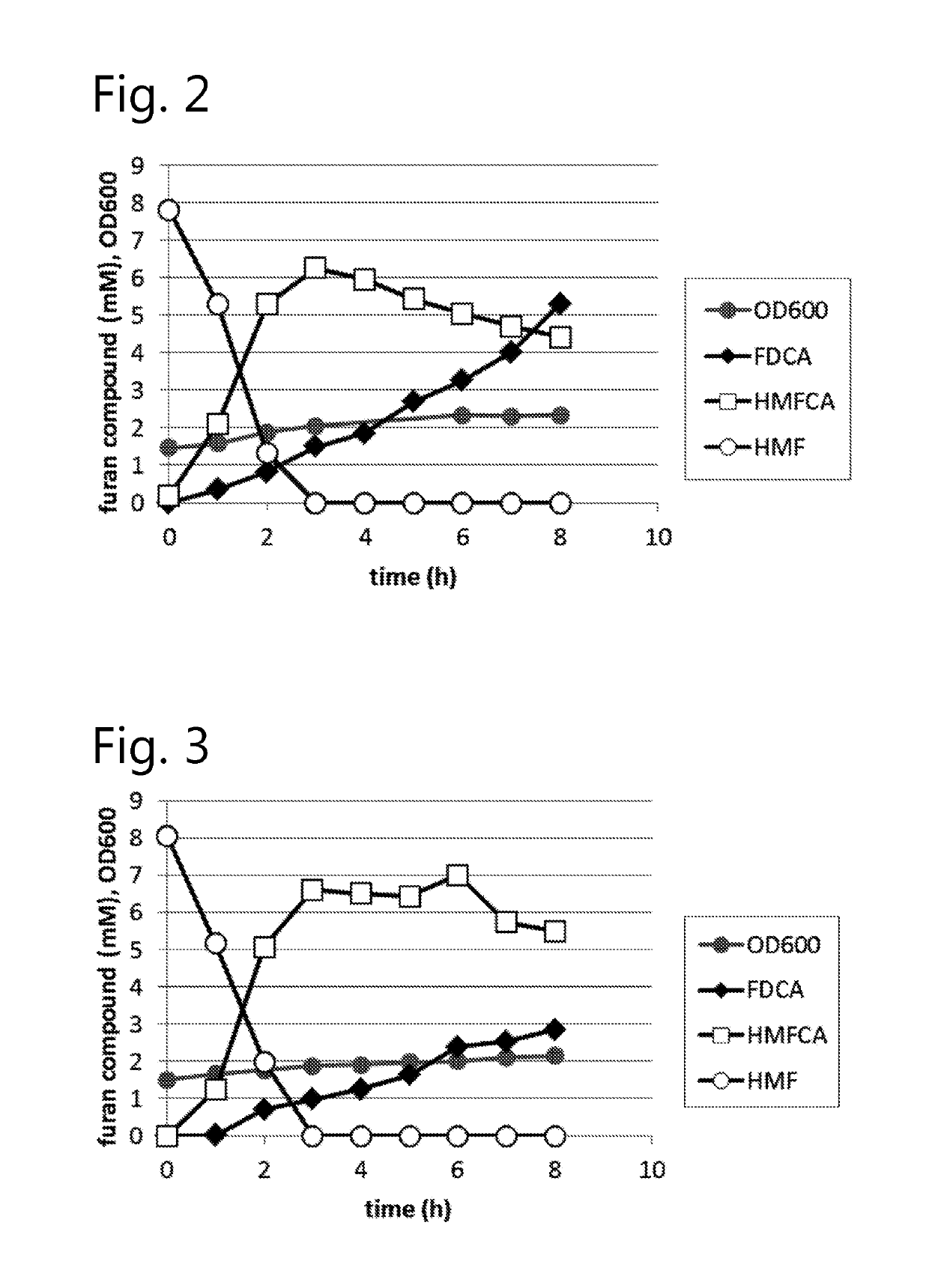
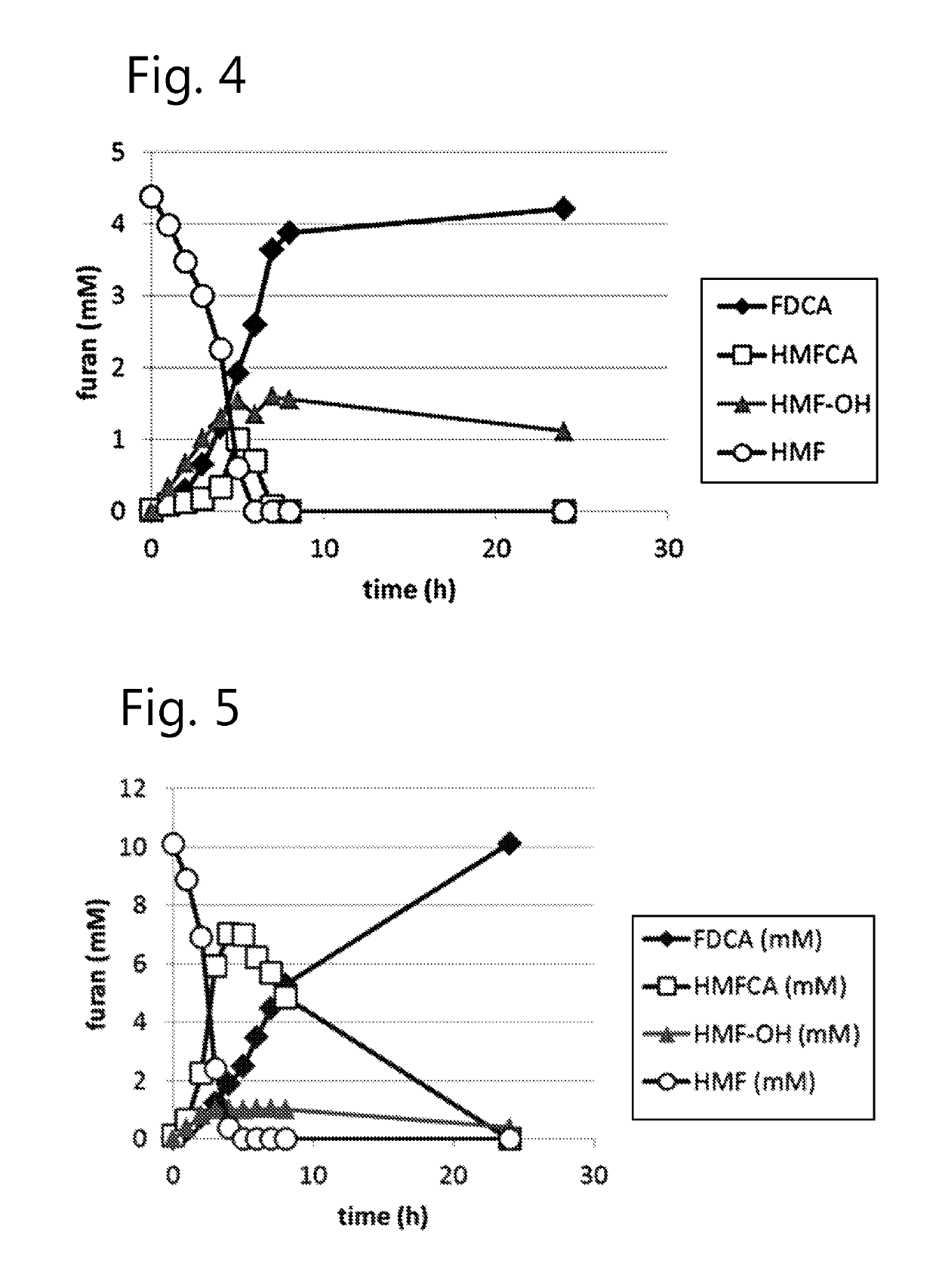
Fungal production of FDCA
The invention relates to fungal cells for the production of FDCA. The fungal cell is genetically modified to have at least one of a) a genetic modification that confers to or increases in the cell the ability to oxidize 5-hydroxymethyl-2-furancarboxylic acid to 5-formyl-2-furoic acid; and, b) a genetic modification that reduces catabolism of 2,5-furandicarboxylic acid in the cell. The fungal cell can further be genetically modified to increase the cell's ability to oxidize furanic aldehydes to the corresponding furanic carboxylic acids. The invention also relates to a process for the production of 2,5-furan-dicarboxylic acid (FDCA) wherein the cells of the invention are used for oxidation of a furanic precursors of FDCA.
Owner:PURAC BIOCHEM
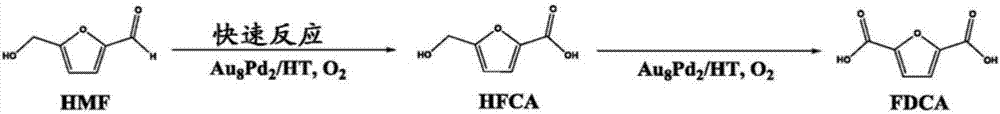
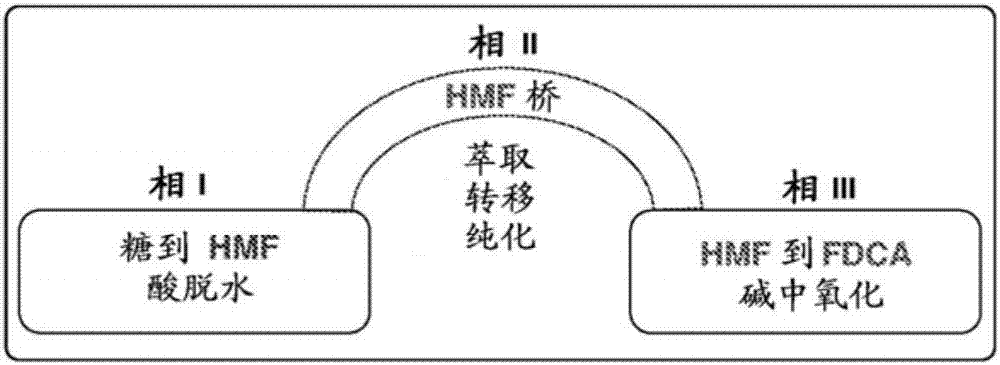
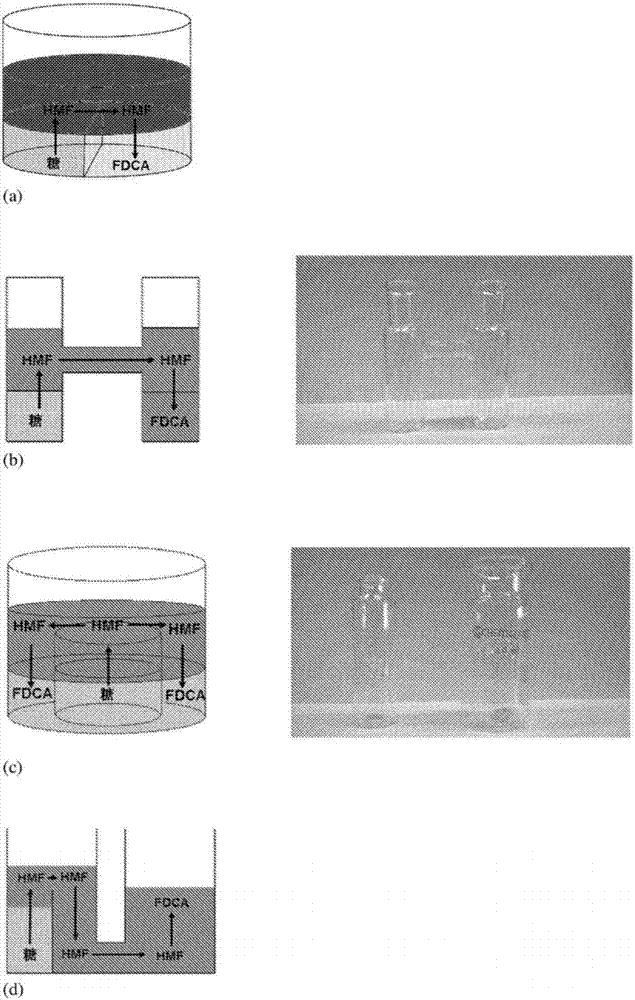
Triphasic system for direct conversion of sugars to furandicarboxylic acid
InactiveCN107001306AShort reaction timeOrganic chemistryChemical/physical/physico-chemical processesTetramethylammonium bromideFuran
There is provided a one-pot process for the conversion of sugars to furancarboxylic acids, such as 2,5-furancarboxylic acid (FDCA), in a triphasic system (e.g. water or tetraethylammonium bromide (TEAB)-methyl isobutyl ketone (MIBK)-water). In this reaction setup, sugars are first converted to 5-hydroxymethylfurfural (HMF) in a first phase. Then HMF is then extracted into a second phase and transferred to a third phase of water. In the third phase HMF is converted to the furancarboxylic acid. The overall acid yields obtainable are between about 78% and 50% for conversion from fructose and glucose, respectively. The invention further relates to an apparatus for the triphasic reaction. The apparatus comprises two chambers which allow for the chemically separated reaction of the sugars and the intermediate of the sugars to form the final product in one process. The process according to the invention may be useful for industrial fabrication.
Owner:AGENCY FOR SCI TECH & RES
Insecticide containing furan compounds and application thereof
The invention discloses an insecticide containing furan compounds and application thereof. The invention discloses an insecticide prepared by taking at least one of 2-furancarboxylic acid, 2-tetrahydrofurancarboxylic acid, (R)-2-tetrahydrofurancarboxylic acid, (S)-2-tetrahydrofurancarboxylic acid, 3-furancarboxylic acid, 3-tetrahydrofurancarboxylic acid, (R)-3-tetrahydrofurancarboxylic acid and (S)-3-tetrahydrofurancarboxylic acid as an active ingredient, and application of the insecticide in killing root-knot nematodes. The active ingredients of the nematicide are derived from microorganismsand plants, are also common industrial raw materials and are easy to degrade in the environment, so that compared with other nematicides, the nematicide has the advantages of high efficiency, low toxicity, low residue and better environmental compatibility, and meets the requirements of green plant protection and sustainable agricultural production and development.
Owner:赵沛基
Insecticide containing furan compounds and application thereof
InactiveCN111226935AHas a strong poisonous effectEasy to degradeBiocideNematocidesFuranMicroorganism
The invention discloses an insecticide containing furan compounds and application of the insecticide, in particular to an insecticide prepared by taking 2-furancarboxylic acid, 2-tetrahydrofurancarboxylic acid and optical isomers thereof as active ingredients. The invention further relates to an application of the insecticide to poisoning and killing root-knot nematodes. The 2-furancarboxylic acidand the 2-tetrahydrofurancarboxylic acid are derived from microorganisms and plants, are also common industrial raw materials, and are easy to degrade in the environment, so that compared with othernematocides, the nematocide has the advantages of high efficiency, low toxicity, low residue and better environmental compatibility, and meets the requirements of green plant protection and sustainable agricultural production and development.
Owner:赵沛基
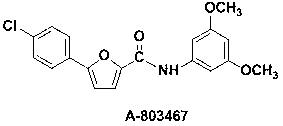
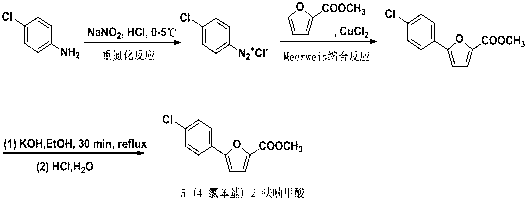
Synthetic method of A-803467 key intermediate
The invention discloses a preparation method of a key intermediate 5-(4-chlorphenyl)-2-furoic acid of a Nav1.8 selective retardant dosage form tool medicine A-803467, relates to a method for preparing 5-(4-chlorphenyl)-2-furoic acid by adopting alkyl nitrite as a diazotization reagent and belongs to the technical field of organic synthesis. The preparation method comprises the following steps of: dissolving parachloroaniline and furoic acid into an organic solvent, slowly dropwise adding alkyl nitrite into a mixed liquor in the presence of a catalyst, keeping a system temperature to be 0-40 DEG C, and carrying out a diazotization reaction and a Meerwein condensation reaction to obtain 5-(4-chlorphenyl)-2-furoic acid. By adopting the preparation method, 5-(4-chlorphenyl)-2-furoic acid can be prepared with high economy and high yield, and yield can be 78%; and reaction selectivity is high, conditions are mild, aftertreatment is simple, and the preparation method is environmentally friendly and applicable to large-scale preparation.
Owner:ZHENGZHOU UNIV
Method for synthesizing 2,5-furandicarboxylic acid from furancarboxylic acid and carbon dioxide
The invention provides a method for synthesizing 2,5-furandicarboxylic acid from furancarboxylic acid and carbon dioxide, and belongs to the technical field of synthesis of 2,5-furandicarboxylic acid. The method comprises the following steps: reacting furancarboxylic acid, inorganic alkali and a solvent under the condition of carbon dioxide to obtain 2, 5-furandimethyl salt, and performing post-treatment to obtain 2, 5-furandicarboxylic acid, wherein the used solvent is an aprotic compound. The aprotic compound is used as the solvent in the reaction process, so that the conversion rate of furancarboxylic acid can be greatly improved, the yield of 2,5-furandicarboxylic acid is increased, and impurities are avoided. Meanwhile, by controlling the reaction conditions, the solubility in the solvent can be increased, and the reaction time is greatly shortened. Experimental results show that the yield of the 2,5-furandicarboxylic acid prepared by the method can reach 99%.
Owner:吉林省中科聚合工程塑料有限公司
Method for synthesizing ceftiofur intermediate and ceftiofur
InactiveCN108912146AReduce manufacturing costHigh yieldOrganic chemistrySolvent7-aminocephalosporanic acid
The invention belongs to the technical field of chemical synthesis, and concretely relates to a method for synthesizing a cefotaxime intermediate 7-amino-3-[(2-furyl-carbonyl)-thiomethyl]-3-cephem-4-carboxylic acid and ceftiofur. The method comprises the following steps: sequentially adding 7-aminocephalosporanic acid, thiofurancarboxylic acid, a solid base catalyst gamma-Al2O2-O2<2->Na<+>, zeolite and water into a reaction kettle, performing stirring and reacting at room temperature for 1.5-2.5 h, filtering the obtained reaction solution, adjusting the pH value of the obtained filtrate to 5.5-6.5 by using an appropriate amount of hydrochloric acid, filtering the filtrate, washing the obtained filter cake with water to obtain a white solid, and performing vacuum drying to obtain the 7-amino-3-[(2-furyl-carbonyl)-thiomethyl]-3-cephem-4-carboxylic acid. The method uses water as a solvent, so the production cost is reduced, the produced products have a high yield and a high purity, and the promotion of industrial production is benefited.
Owner:SHANDONG JIULONG HISINCE PHARMA CO LTD
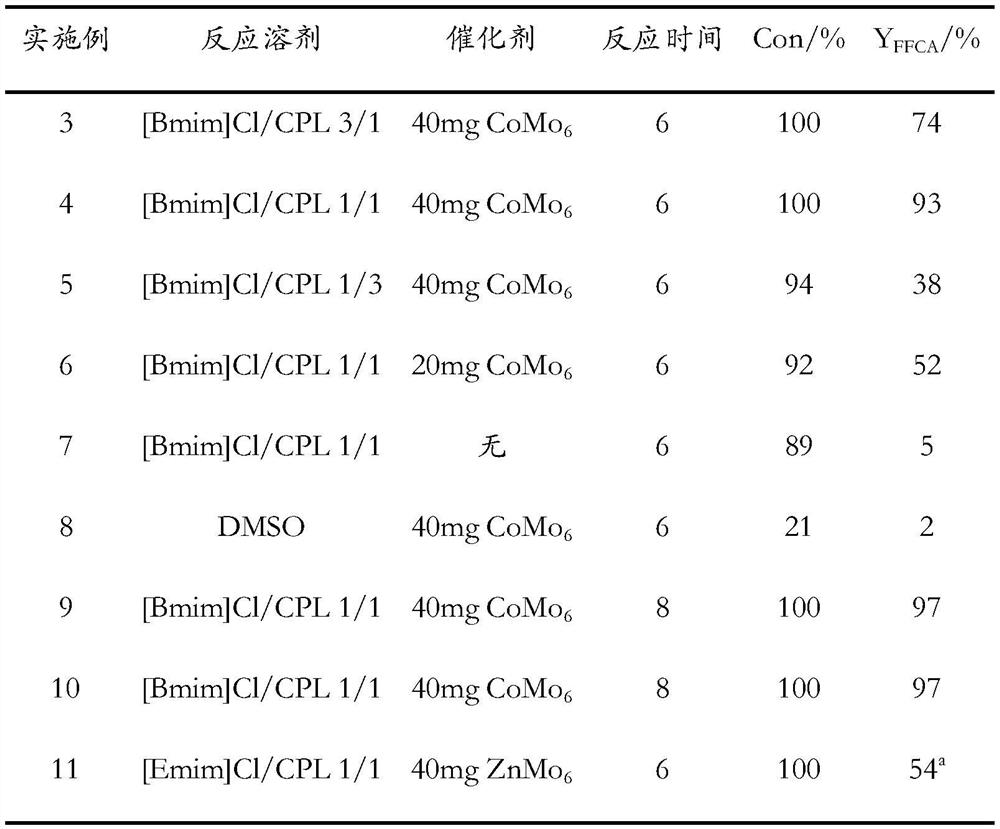
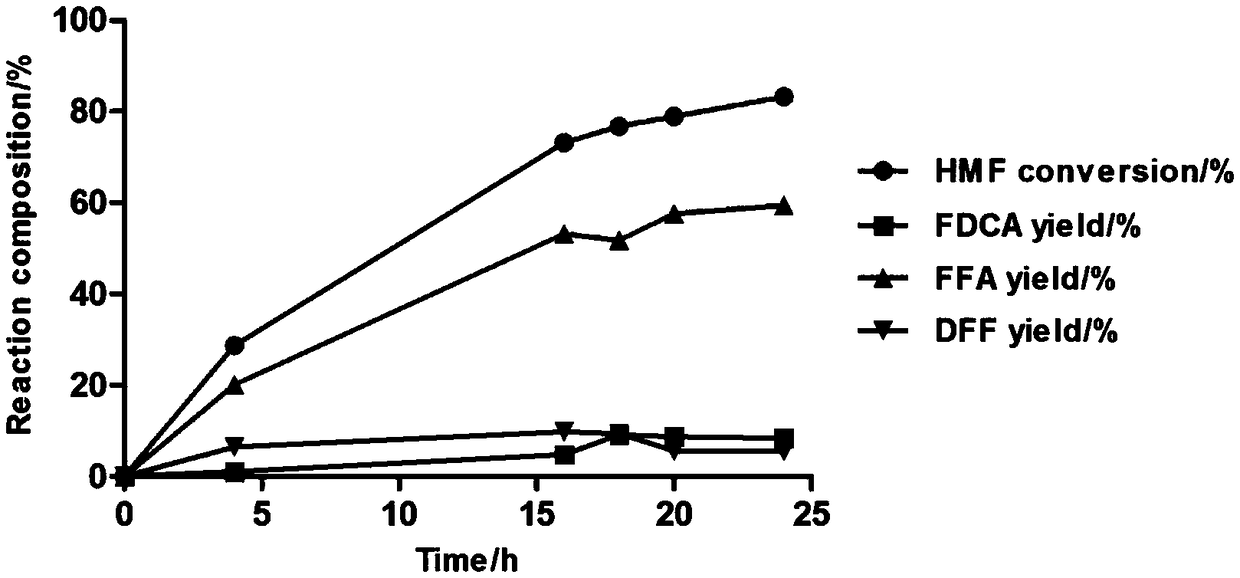
Method for preparing 5-formyl-2-furancarboxylic acid through catalytic oxidation of 5-hydroxymethylfurfural
ActiveCN113845500AImprove catalytic performanceEfficient synthesisOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranPtru catalyst
The invention discloses a method for preparing 5-formyl-2-furancarboxylic acid through catalytic oxidation of 5-hydroxymethylfurfural. The method specifically comprises the following steps: dissolving 5-hydroxymethylfurfural and an Anderson type polyoxometallate catalyst in a deep eutectic solvent, introducing oxygen at a specified temperature, and carrying out a reaction to obtain the 5-formyl-2-furancarboxylic acid. According to the above technical process, the 5-formyl-2-furancarboxylic acid (with yield reaching 97%) can be efficiently synthesized, reaction conditions are mild, and the method is a cheap and efficient FFCA preparation method. Meanwhile, the technological process is easy to operate, high in selectivity and low in cost, reference is provided for renewable biomass resource conversion, and the method has important significance on development of novel renewable energy sources.
Owner:YANTAI UNIV
Furoic acid beta-caryophyllene-5-ester compound as well as preparation method and application of furoic acid beta-caryophyllene-5-ester compound
ActiveCN110804033AThe reaction steps are simpleMild reaction conditionsOrganic chemistryAntipyreticFuranCytotoxicity
The invention discloses a furancarboxylic acid beta-caryophyllene-5-ester compound as well as a preparation method and application thereof, and belongs to the technical field of preparation of carboxylic acid beta-caryophyllenol ester. The preparation method of the furoic acid beta-caryophyllene-5-ester compound comprises the following steps: reacting furoic acid with DCC, adding beta-caryophyllenol and DMAP, and reacting to obtain the compound after the reaction is finished. NO inhibition rate experiments, cytotoxicity experiments and anti-cancer activity experiments prove that the compoundshave a certain inhibition effect on inflammation, have good anti-cancer activity on cervical cancer, liver cancer, breast cancer or lung cancer, and can be applied to preparation of anti-inflammatoryand anti-cancer drugs.
Owner:NANJING FORESTRY UNIV
Dehydrogenase-catalysed production of FDCA
InactiveUS10457965B2Raise the possibilityImprove usabilityBacteriaOxidoreductasesFuranDicarboxylic acid
The invention relates to a cell expressing a polypeptide having 5-hydroxymethyl-2-furancarboxylic acid dehydrogenase activity, as well as to a cell expressing a polypeptide having furanic compound transport capabilities. The invention also relates to a process for the production of 2,5-furan-dicarboxylic acid (FDCA) wherein the cells of the invention are used for oxidation of a furanic precursors of FDCA.
Owner:PURAC BIOCHEM
Method for producing furan ammonium salt by using furoic acid
The invention discloses a method for producing a furan ammonium salt by using furoic acid. The method comprises the following steps of: (1) chlorination: putting furoic acid and chlorohydrocarbon solvent into an enameled glass reaction kettle, controlling temperature and time, and obtaining a furoyl chloride product; (2) cyanation: putting the furoyl chloride and sodium cyanide into the reaction kettle, stirring, preserving heat, reducing pressure, and reclaiming the solvent to obtain a furoyl nitrile product; (3) hydrolysis: heating the furoyl nitrile and hydrochloric acid, and obtaining furan ketone acid after the reaction is finished; (4) condensation: adding ethyl acetate extract of the furan ketone acid serving as the hydrolysis product into the reaction kettle, dropping methoxyamineto perform room temperature reaction with the hydrolysis product, and demixing, wherein the oil layer is used for ammoniation reaction; and (5) ammoniation: introducing ammonia gas into the oil layerreactant, controlling the temperature, stirring, finishing gas introduction, centrifuging, and drying to obtain the furan ammonium salt. The method is simple and convenient, is convenient to operate,reduces the production cost, improves the yield, makes full use of raw materials, and is safe and reliable in the production process.
Owner:湖北楚阳科技股份有限公司
New application of bis(α-furanoic acid)vanadyl as an anticancer drug
InactiveCN103599107BStrong inhibitory activityLow toxicityOrganic active ingredientsAntineoplastic agentsFuranCancer cell
Owner:KUNMING INST OF PRECIOUS METALS +2
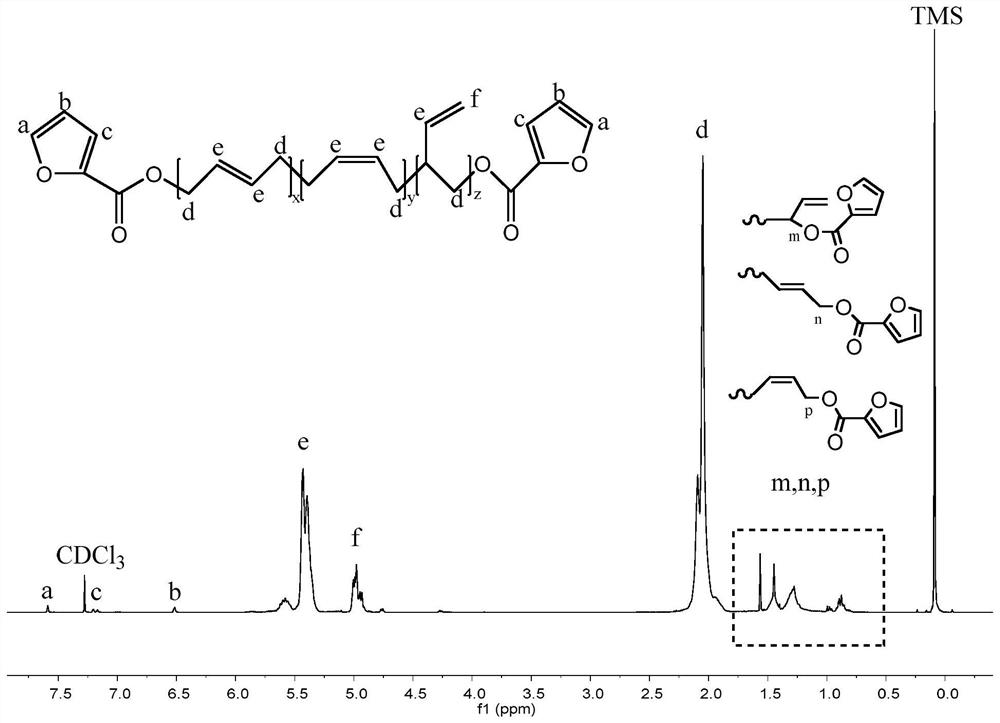
A kind of method for preparing terminal 2-furanoate group polybutadiene
ActiveCN112625151BGood catalyticImprove the efficiency of esterification reactionFuranPolymer science
The invention provides a method for preparing polybutadiene with 2-furanoic acid ester group, comprising the following steps: adding hydroxyl-terminated polybutadiene, 4-dimethylaminopyridine and an organic solvent into a reactor, stirring at room temperature fully Mix well; cool the mixture to 0°C, slowly add 2-furoyl chloride, then stir the reaction, the reaction temperature is 0°C~room temperature, and the reaction time is 1h~48h; the mixture is washed, dried, concentrated organic phase, and separated and purified by column chromatography Obtain product terminal 2-furanoate group polybutadiene. In the present invention, while using 4-dimethylaminopyridine as an acid-binding agent, the efficiency of the esterification reaction can be effectively improved, and the 2-furoyl chloride has high reactivity and low cost, and avoids the use of dicyclohexylcarbodiimide. The reaction operation and post-treatment in the invention are simple and safe, the yield is stable in the scale-up preparation process, and has great industrial application potential.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
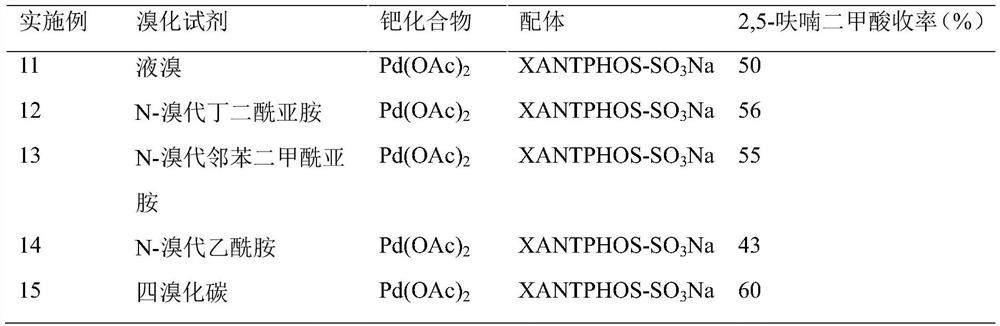
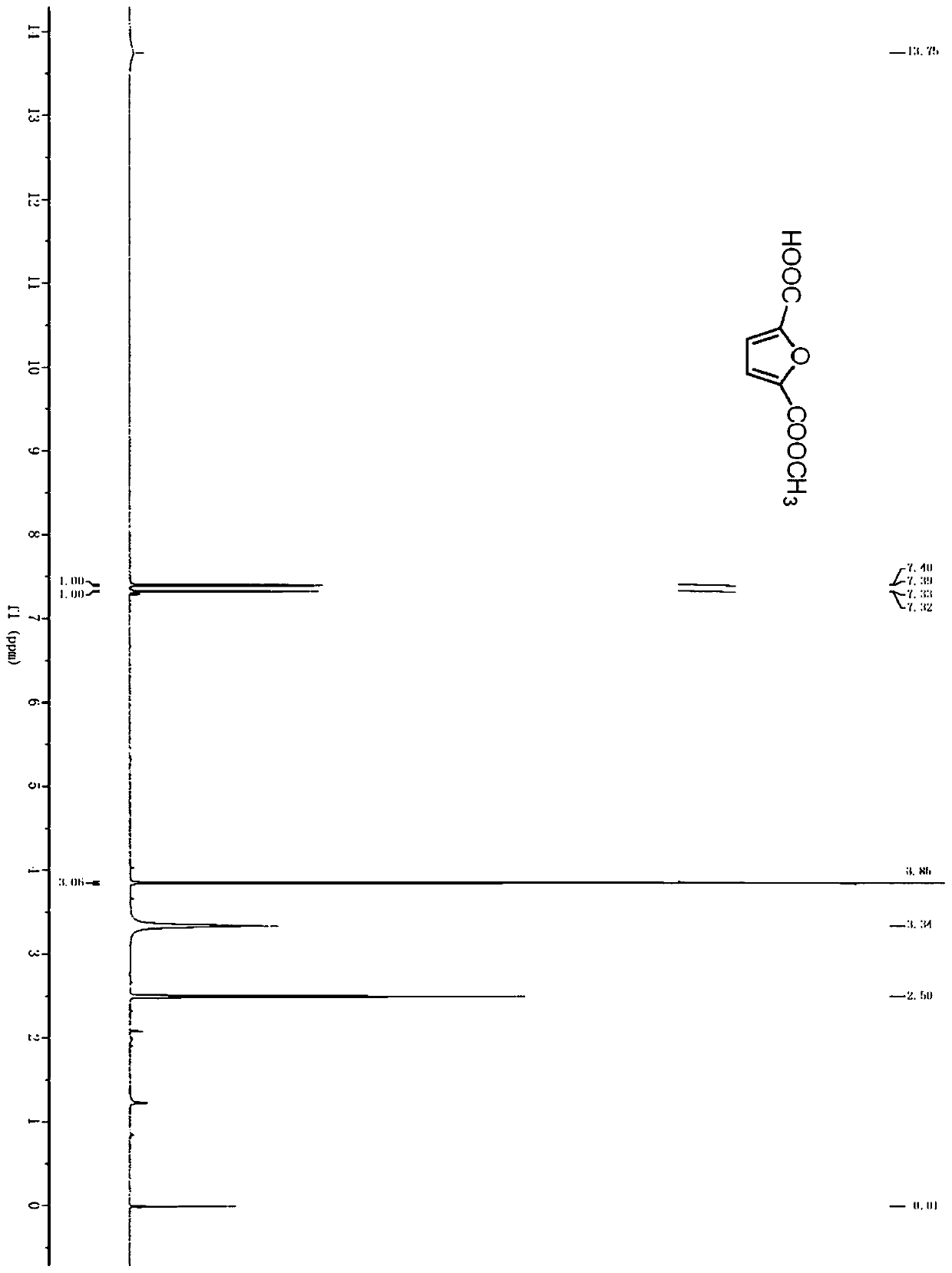
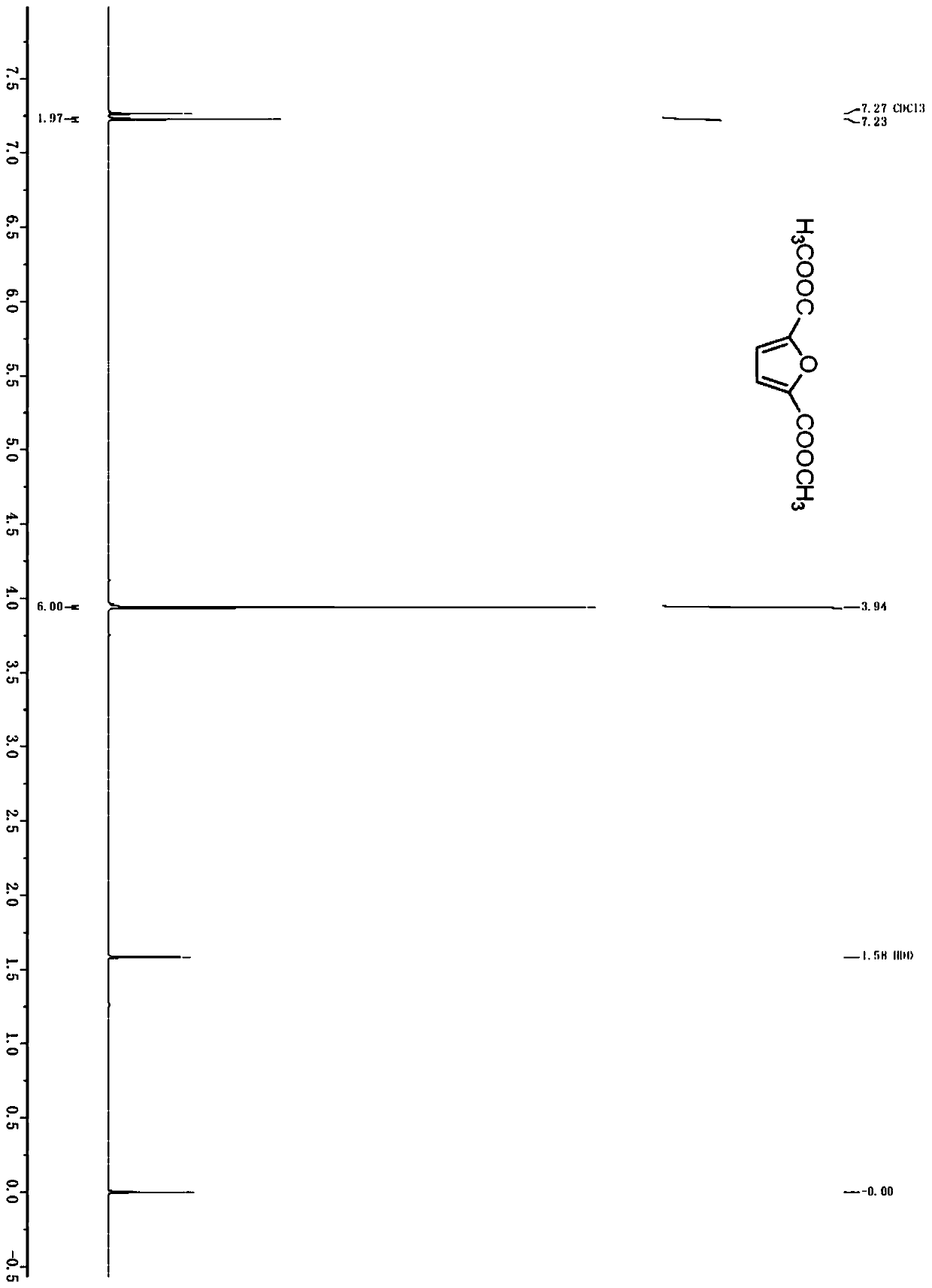
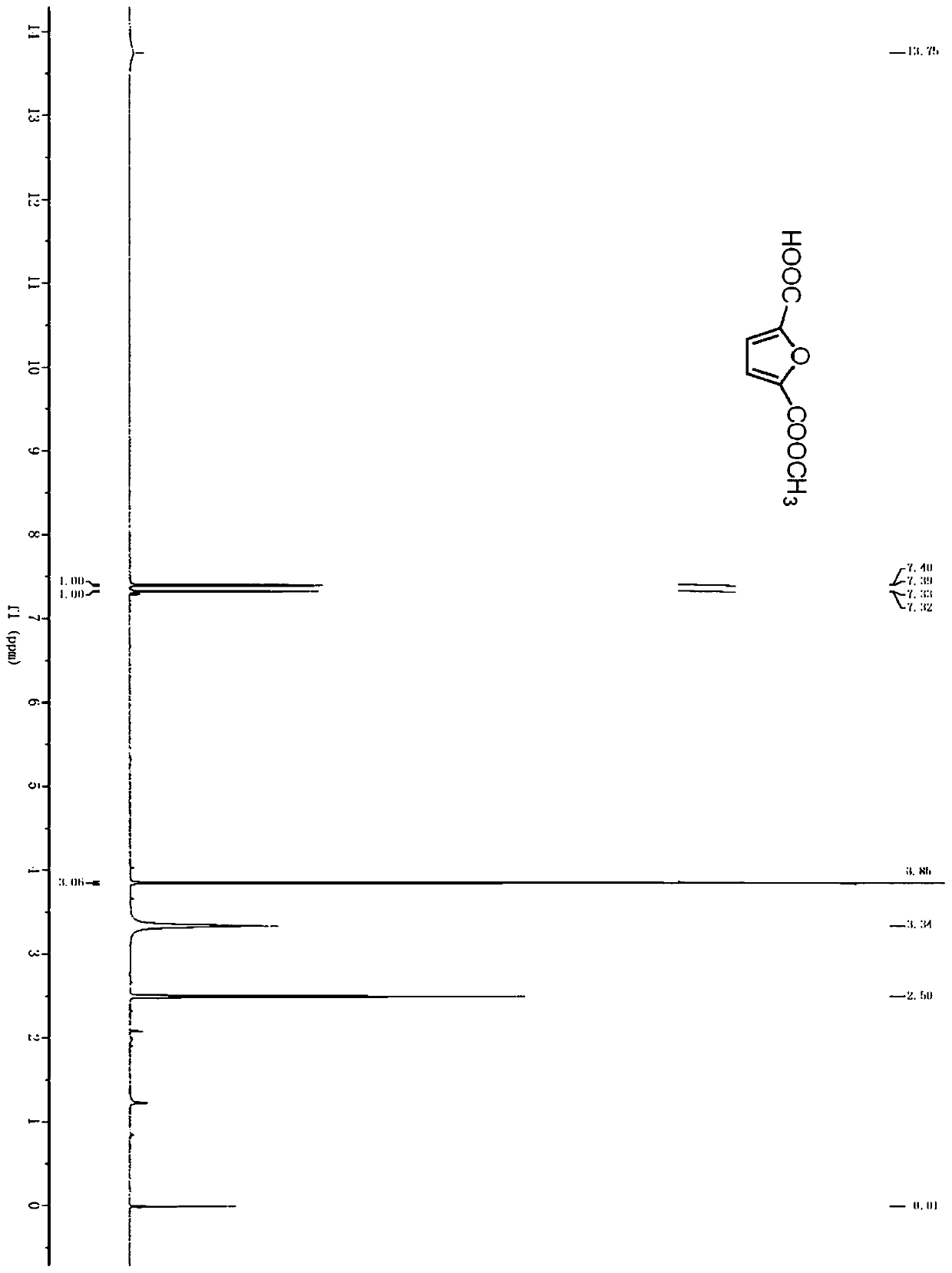
Method for preparing 2, 5-furandicarboxylic acid by taking 2-furancarboxylic acid as raw material through one-pot method
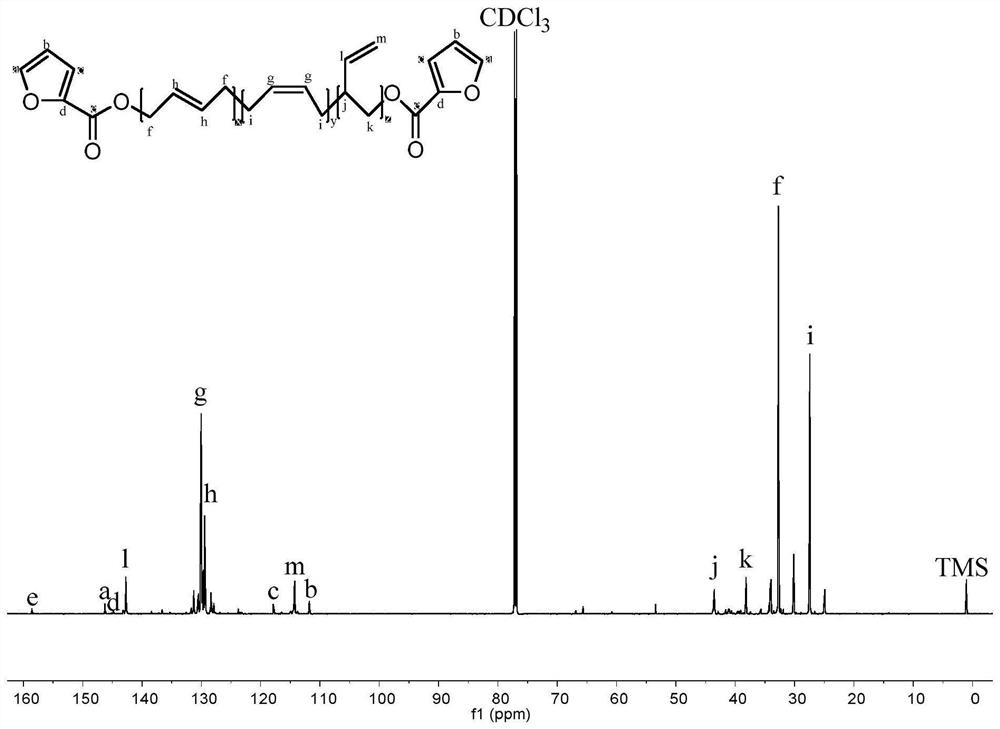
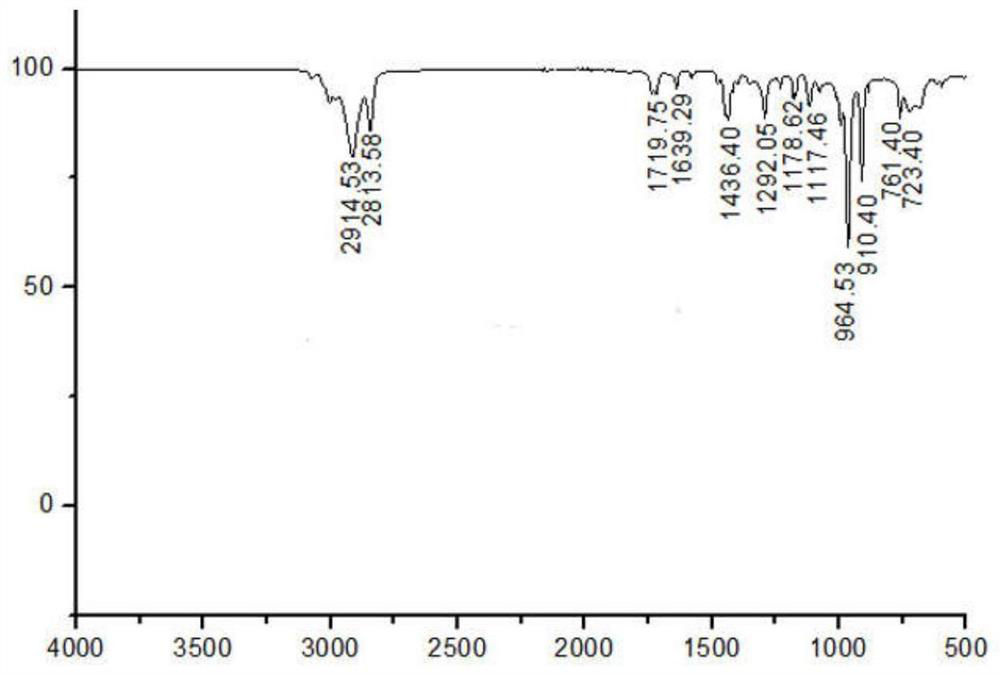
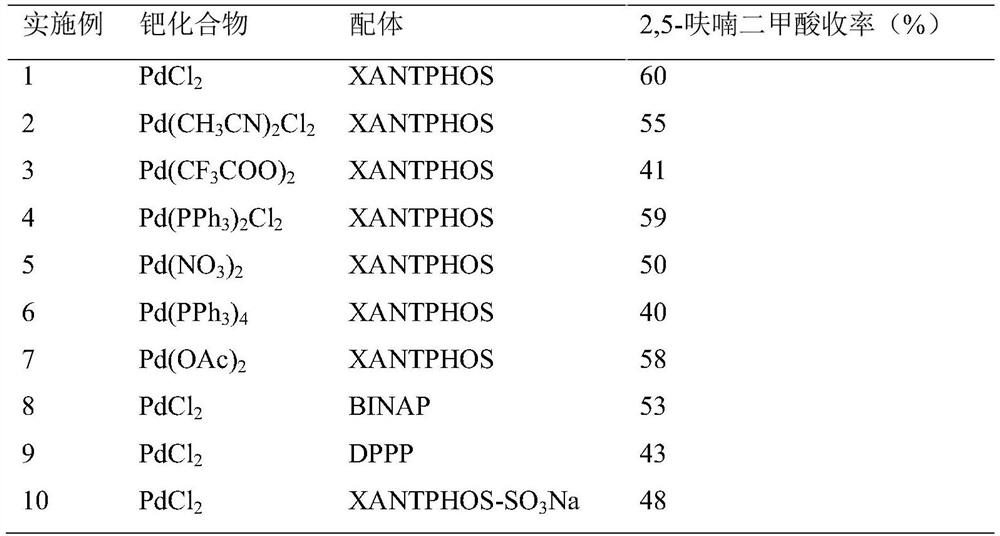
ActiveCN114085200AEnvironmentally friendlyHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranPtru catalyst
The invention discloses a method for preparing 2, 5-furandicarboxylic acid by taking 2-furancarboxylic acid as a raw material through an one-pot method, which comprises the following steps of: stirring and reacting a solution system containing 2-furancarboxylic acid, a solvent, a bromination reagent, a catalyst and a buffer solution in a carbon monoxide atmosphere at the temperature of 50-150 DEG C for 4-24 hours by taking 2-furancarboxylic acid as the raw material under the action of a catalyst; and then separating out the target product 2, 5-furandicarboxylic acid. The 2, 5-furandicarboxylic acid is prepared from the biomass-derived 2-furancarboxylic acid as a raw material through the one-pot bromination-carbonylation cascade reaction, the synthesis process is a one-pot synthesis technology, the synthesis steps are simple, and the 2, 5-furandicarboxylic acid is directly obtained without acidification after the reaction is finished.
Owner:EAST CHINA NORMAL UNIV
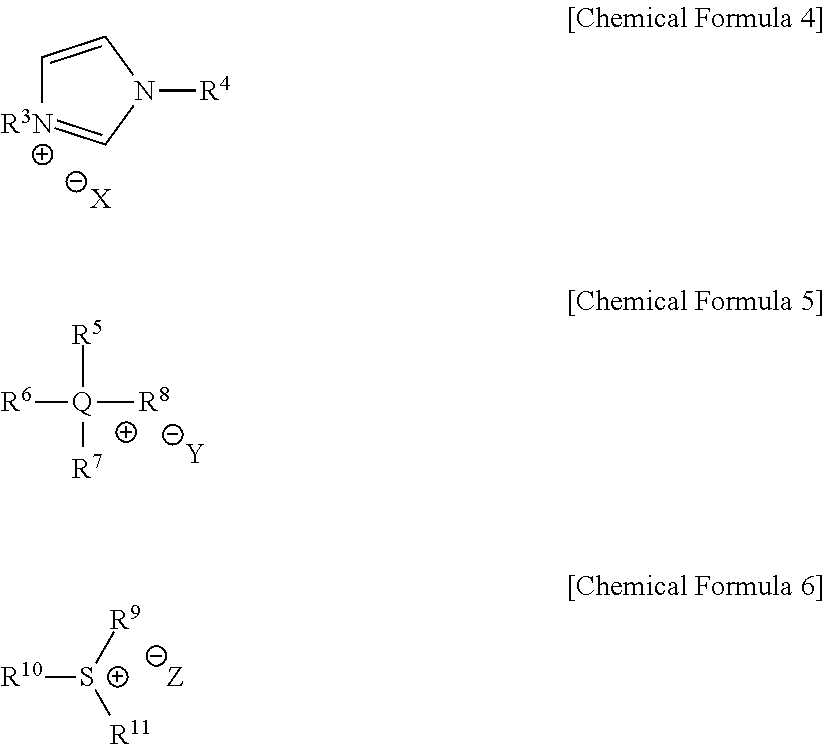
Method for preparing a furfuranol-based compound and 2-furancarboxylic acid-based compound using an ionic liquid as a solvent
Owner:KOREA INST OF IND TECH +1
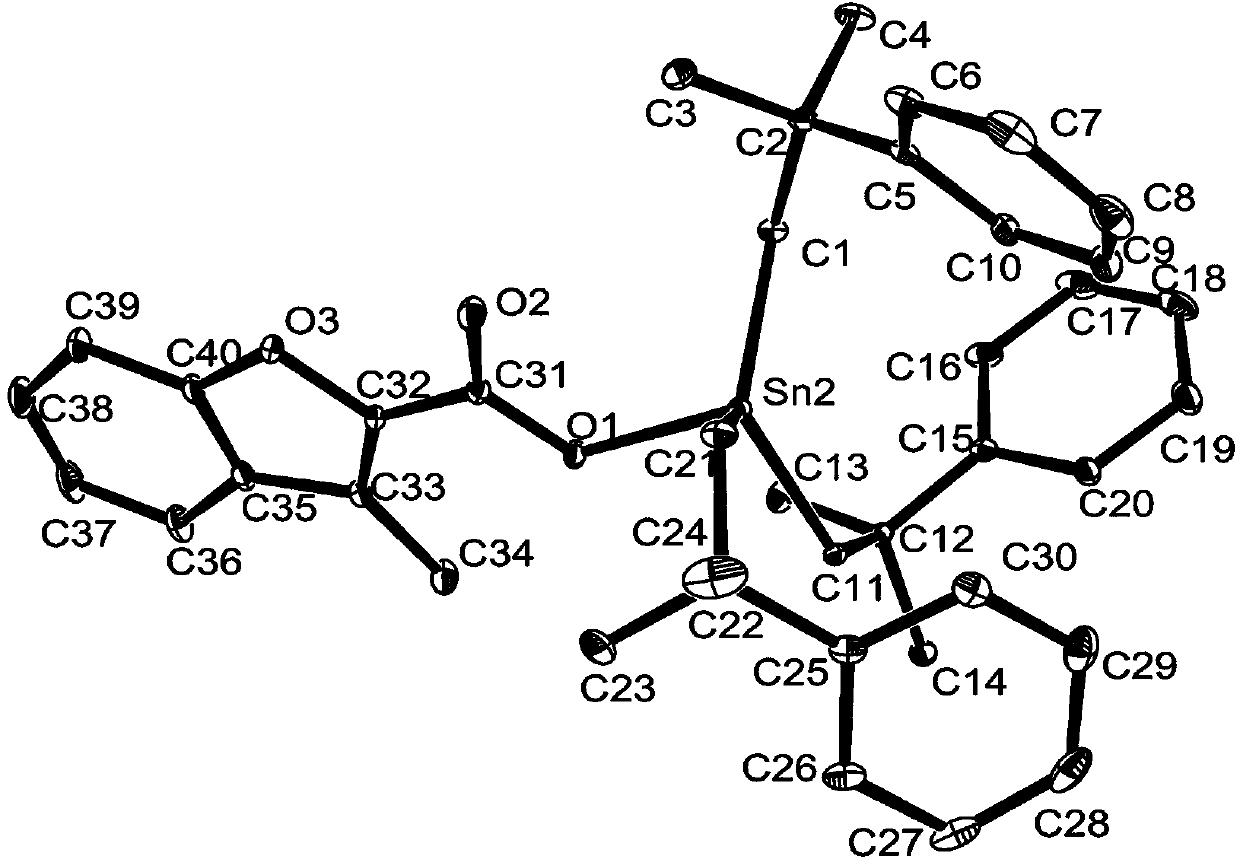
Tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex, and preparation method and application thereof
ActiveCN111138475AHigh anticancer activityLow costOrganic active ingredientsTin organic compoundsPolymer scienceMethyl benzene
The invention discloses a tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex, and a preparation method and an application thereof. The tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex is a complex represented by structural formula (I) shown in the description. The invention further discloses the preparation method of the tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex and the application of the tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex in the preparation of antitumor drugs.
Owner:HENGYANG NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
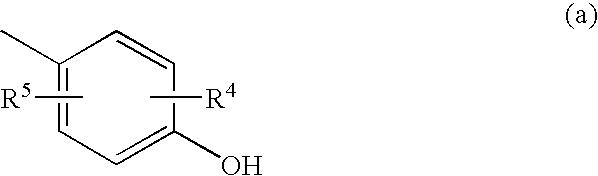
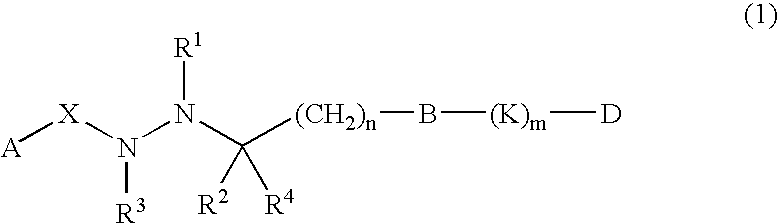


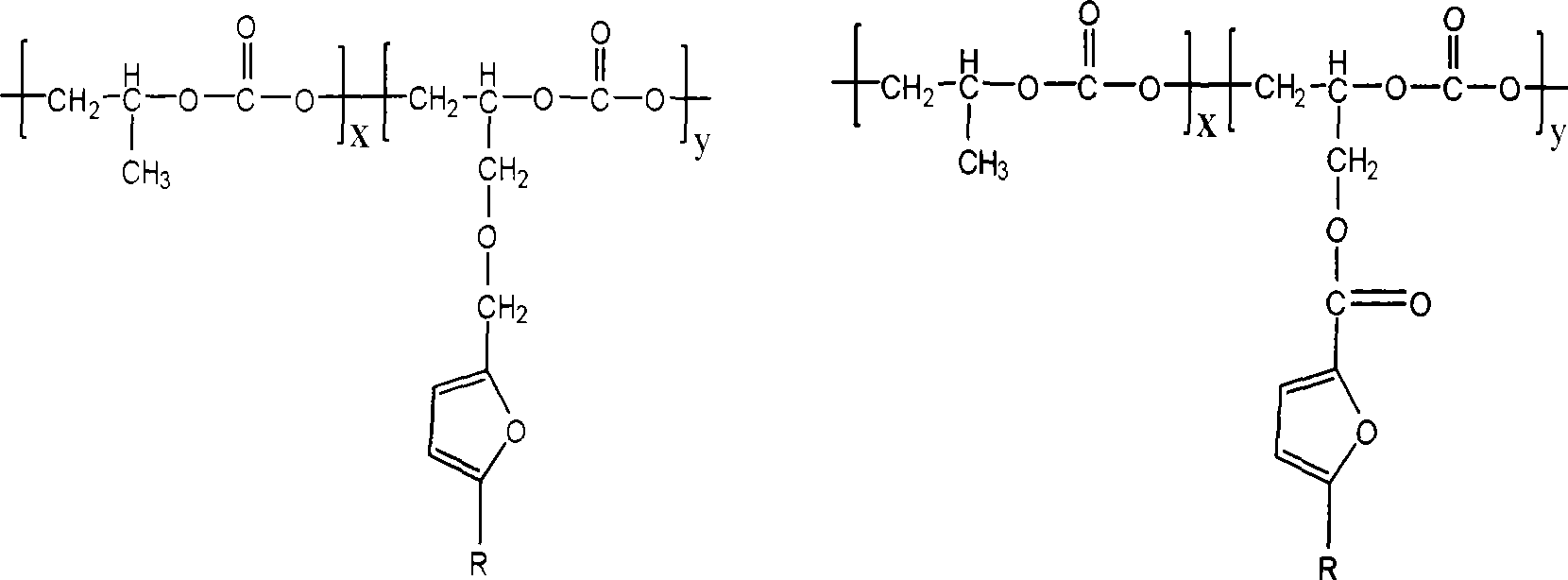
![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka.patsnap.com/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/2014107721379100002DEST_PATH_IMAGE001.PNG)
![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka.patsnap.com/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/309262DEST_PATH_IMAGE002.PNG)
![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka.patsnap.com/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/657249DEST_PATH_IMAGE001.PNG)