Insecticide containing furan compounds and application thereof
A technology of pesticides and compounds, applied in the field of pesticides, to achieve the effect of good compatibility and easy degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Study on the poisonous activity of 2-furancarboxylic acid against Meloidogyne incognita
[0016] (1) Sample preparation
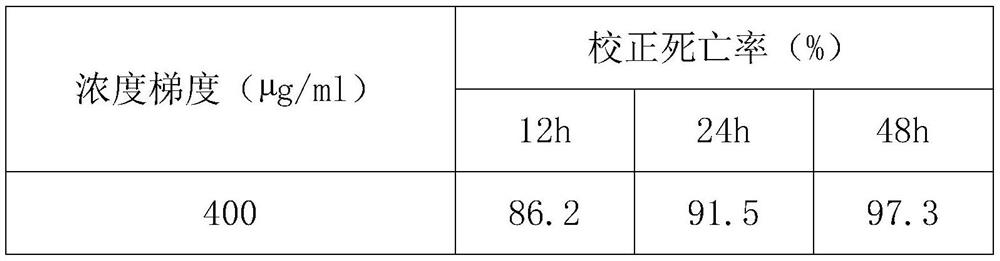
[0017] Dissolve 0.5mg-1.5mg of 2-furancarboxylic acid in methanol, dilute the concentration with sterile distilled water, and prepare five concentration gradients of 25-400μg / ml, and the final concentration of methanol is no more than 5%.
[0018] (2) Preparation of nematodes for testing
[0019] Wash the tomato roots with Meloidogyne incognita cultivated in the greenhouse with sterile water, pick out the oocysts of the root-knot nematode with a dissecting needle and place them in 0.4M KCl solution, and use 4ml of no Bacteria water (three times), 1ml of sterile penicillin-streptomycin double antibody solution, 3ml of 10.1% NaClO solution for washing and disinfection, each step was briefly centrifuged (8000 rpm for 30 seconds) to remove the supernatant. The washed eggs were placed in 0.4M ZnCl 2 After hatching in the solution for 48 hours, the seco...
Embodiment 2
[0031] Study on the Toxic Activity of 2-Tetrahydrofurancarboxylic Acid and Its Isomers (R)-2-Tetrahydrofurancarboxylic Acid and (S)-2-Tetrahydrofurancarboxylic Acid Mixtures Against Meloidogyne japonicum
[0032] This example is basically the same as the experimental method used in Example 1, except that the test compound is replaced by 2-tetrahydrofuran formic acid and its isomers (R)-2-tetrahydrofuran formic acid, (S)-2- A mixture of tetrahydrofurancarboxylic acid; the nematodes used in the experiment were replaced by Meloidogyne incognita derived from tomato roots and Meloidogyne incognita derived from Nicotiana nicotianae. The obtained experimental results are as follows:
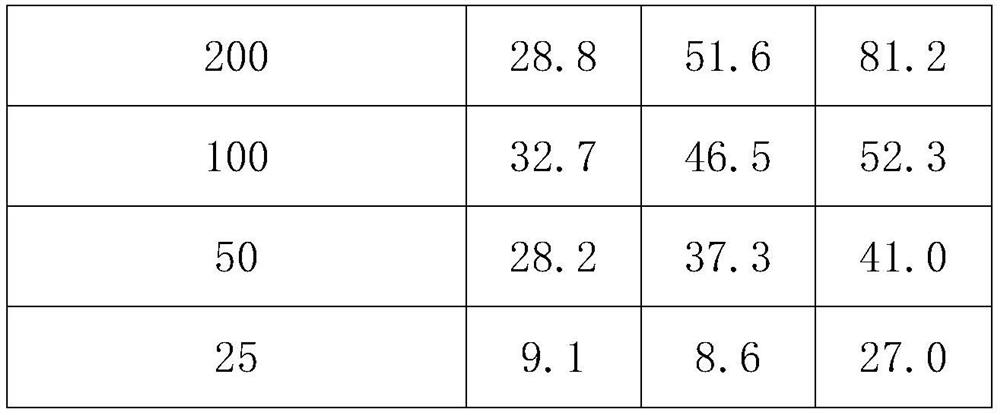
[0033] Table 2 The poisoning results of 2-tetrahydrofurancarboxylic acid and its isomer mixture to root-knot nematode northern root-knot nematode (%)
[0034]
[0035]
[0036] As can be seen from Table 2: the mixture of 2-furan formic acid and its isomer (R)-2-tetrahydrofuran formic acid and (S)...
Embodiment 3
[0038] Study on the Toxic Activity of 3-Furancarboxylic Acid to Meloidogyne javanica
[0039] (1) Sample preparation
[0040] Dissolve 3-furancarboxylic acid in methanol, dilute the concentration with sterile distilled water, prepare five concentration gradients of 50-800 μg / ml, and the final concentration of methanol does not exceed 5%.
[0041] (2) Preparation of nematodes for testing
[0042] Meloidogyne javanica was collected from cucumbers planted by farmers, and the root nodules infected with root-knot nematodes were taken, washed with sterile water, and the oocysts on the root nodules were picked with a dissecting needle and placed in 0.4M KCl solution, and placed in a 5ml centrifuge tube Wash and disinfect with 4ml sterile water (three times), 1ml sterile penicillin-streptomycin double antibody solution, and 3ml 0.1% NaClO solution, and remove the supernatant by brief centrifugation (8000 rpm for 30 seconds) in each step. The washed eggs were placed in 0.4M ZnCl 2 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com