Insecticide containing furan compounds and application thereof
A technology of insecticides and compounds, applied in the field of insecticides, can solve the problems of no nematicide activity and other problems, and achieve the effects of strong poisonous killing effect, good environmental compatibility, and easy degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: The poisonous activity of 2-furancarboxylic acid to root-knot nematode incognita
[0015] 1. Sample preparation
[0016] Dissolve 0.5 mg-1.5 mg of 2-furancarboxylic acid in methanol, dilute the concentration with sterile distilled water, and the final concentration of methanol should not exceed 5%.
[0017] 2. Preparation of nematodes for experiment
[0018] Wash the tomato roots with Meloidogyne incognita cultivated in the greenhouse with sterile water, pick out the oocysts of the root-knot nematode with a dissecting needle and place them in 0.4M KCl solution, and use 4ml of no Bacteria water (three times), 1ml of sterile penicillin-streptomycin double antibody solution, 3ml of 10.1% NaClO solution for washing and disinfection, each step was briefly centrifuged (8000 rpm for 30 seconds) to remove the supernatant. The washed eggs were placed in 0.4M ZnCl 2 After incubation in the solution for 48 hours, the second-instar larvae were collected in a 1.5ml ...
Embodiment 2
[0028] Example 2: Toxic activity of mixtures of 2-tetrahydrofurancarboxylic acid and its isomers (R)-2-tetrahydrofurancarboxylic acid and (S)-2-tetrahydrofurancarboxylic acid against root-knot nematode northern
[0029] The experimental method used in this example is basically the same as in Example 1, except that the test compound 2-furan formic acid is replaced by 2-tetrahydrofuran formic acid and its isomers (R)-2-tetrahydrofuran formic acid, (S)-2-tetrahydrofuran A mixture of formic acid; the test nematodes were replaced by M. incognita. The obtained experimental results are as follows:
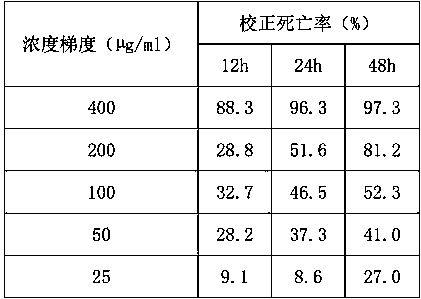
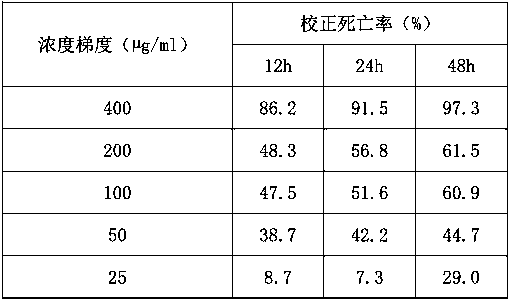
[0030] Table 2 The poisoning results of 2-tetrahydrofurancarboxylic acid and its isomer mixtures on root-knot nematodes (%)
[0031]
[0032] As can be seen from Table 2: the mixture of 400 µg / ml 2-furan formic acid and its isomers (R)-2-tetrahydrofuran formic acid and (S)-2-tetrahydrofuran formic acid is effective against root-knot nematode (Meloidogyne nematode) in 48 hours. hapla)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com