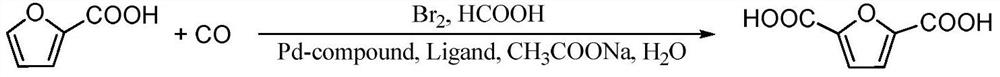Method for preparing 2, 5-furandicarboxylic acid by taking 2-furancarboxylic acid as raw material through one-pot method
A technology of furandicarboxylic acid and furanic acid, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as poor purity, achieve mild reaction conditions, The effect of a simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
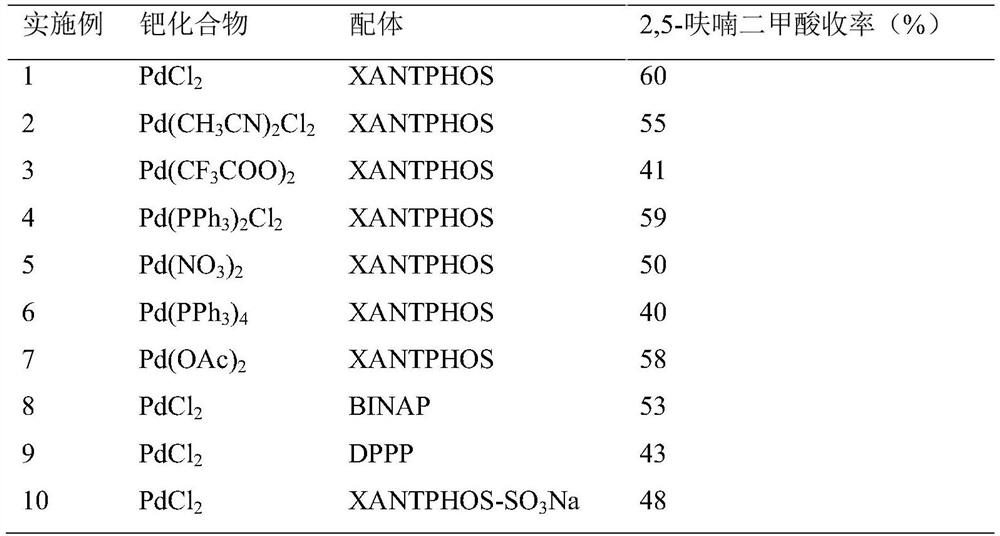
[0019] (1) Effects of catalysts composed of different palladium compounds and ligands on the preparation of 2,5-furandicarboxylic acid from 2-furancarboxylic acid
[0020] Specific experimental steps: Add 5mL of N-methylpyrrolidone solvent, 2.5mL of deionized water, 5mmol of 2-furanoic acid, 0.25mmol of palladium compound, 0.25mmol of ligand, and 10mmol of liquid into a 50mL polytetrafluoroethylene-lined stainless steel autoclave. bromine, 15 mmol sodium acetate and 2.5 mL formic acid. After sealing, replace the air in the reactor with nitrogen, then flush into 1.0 MPa of carbon monoxide, and react at 90°C for 9 hours. After the reaction, cool to room temperature, and calculate 2,5-furan by HPLC (high performance liquid chromatography). The yield of formic acid.
[0021]
[0022]
[0023] Note: XANTPHOS, 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene; BINAP, 1,1'-binaphthyl-2,2'-bisdiphenylphosphine; DPPP, 1 ,3-bis(diphenylphosphino)propane; XANTPHOS-SO 3 Na, water-s...
Embodiment 11-15
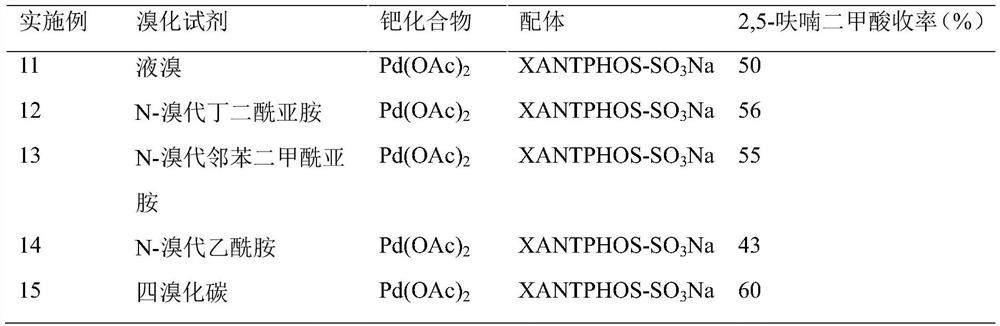
[0025] (2) Effects of different bromination reagents on the preparation of 2,5-furandicarboxylic acid from 2-furancarboxylic acid
[0026] Specific experimental steps: Add 5mL of N-methylpyrrolidone solvent, 2.5mL of deionized water, 5mmol of 2-furanoic acid, 0.25mmol of palladium acetate, and 0.25mmol of water-soluble ligand into a 50mL polytetrafluoroethylene-lined stainless steel autoclave XANTPHOS-SO 3Na, 10mmol brominating reagent, 15mmol sodium acetate and 2.5mL formic acid. After sealing, replace the air in the reactor with nitrogen, then flush into 1.0 MPa of carbon monoxide, and react at 90°C for 9 hours. After the reaction, cool to room temperature, and calculate 2,5-furan by HPLC (high performance liquid chromatography). The yield of formic acid.
[0027]
[0028] Note: XANTPHOS, 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene; BINAP, 1,1'-binaphthyl-2,2'-bisdiphenylphosphine; DPPP, 1 ,3-bis(diphenylphosphino)propane; XANTPHOS-SO 3 Na, water-soluble 4,5-bis(d...
Embodiment 16-21
[0030] (3) Effects of different buffers on the preparation of 2,5-furandicarboxylic acid from 2-furancarboxylic acid
[0031] Specific experimental steps: Add 5mL of organic solvent, 2.5mL of deionized water, 15mmol of various sodium salts, 2.5mL of various acids, 5mmol of 2-furancarboxylic acid, 0.25mmol of Palladium chloride, 0.25mmol water-soluble ligand XANTPHOS-SO 3 Na and 10mmol liquid bromine. After sealing, replace the air in the reactor with nitrogen, then flush into 1.0 MPa of carbon monoxide, and react at 90°C for 9 hours. After the reaction, cool to room temperature, and calculate 2,5-furan by HPLC (high performance liquid chromatography). The yield of formic acid.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com