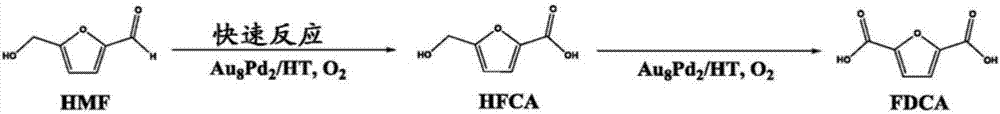
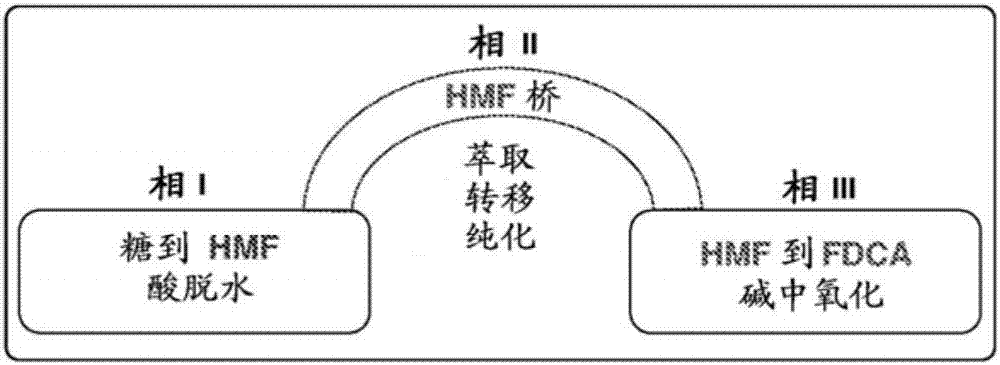
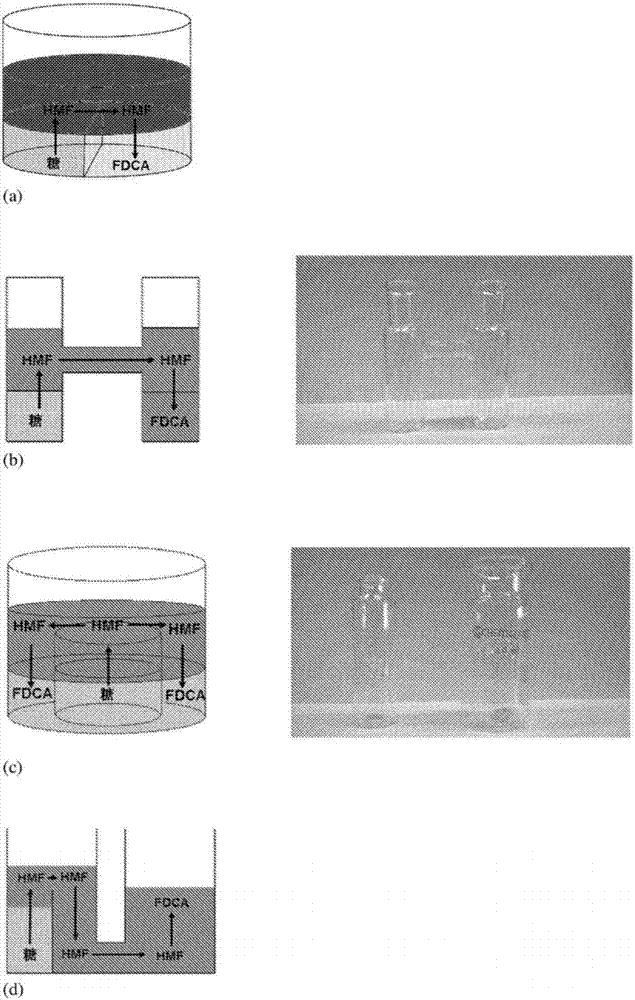
Triphasic system for direct conversion of sugars to furandicarboxylic acid
A furandicarboxylic acid, conversion technology, applied in the direction of organic chemistry, chemical instruments and methods, chemical/physical processes, etc., to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] An exemplary, non-limiting embodiment of the one-pot process will now be disclosed.
[0032] Provided is a one-pot process for producing furandicarboxylic acid from carbohydrates, the process comprising:
[0033] a) reacting said carbohydrate via a dehydration reaction to produce an intermediate in a first solvent phase; b) contacting said first solvent phase at a first contact zone with a second solvent phase at a first contact zone; c) extracting the intermediate into the second solvent phase; d) contacting the second solvent phase directly with a third solvent phase at a different contact zone; and e) oxidizing the intermediate to The furandicarboxylic acid is produced in the third solvent phase.
[0034]In step a), said carbohydrate may be a carbohydrate capable of being dehydrated to said intermediate. The carbohydrates may preferably be selected from cellulose, fructose, glucose, any other sugar, or isomers thereof, such as D-glucose or D-fructose. Fructose and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com