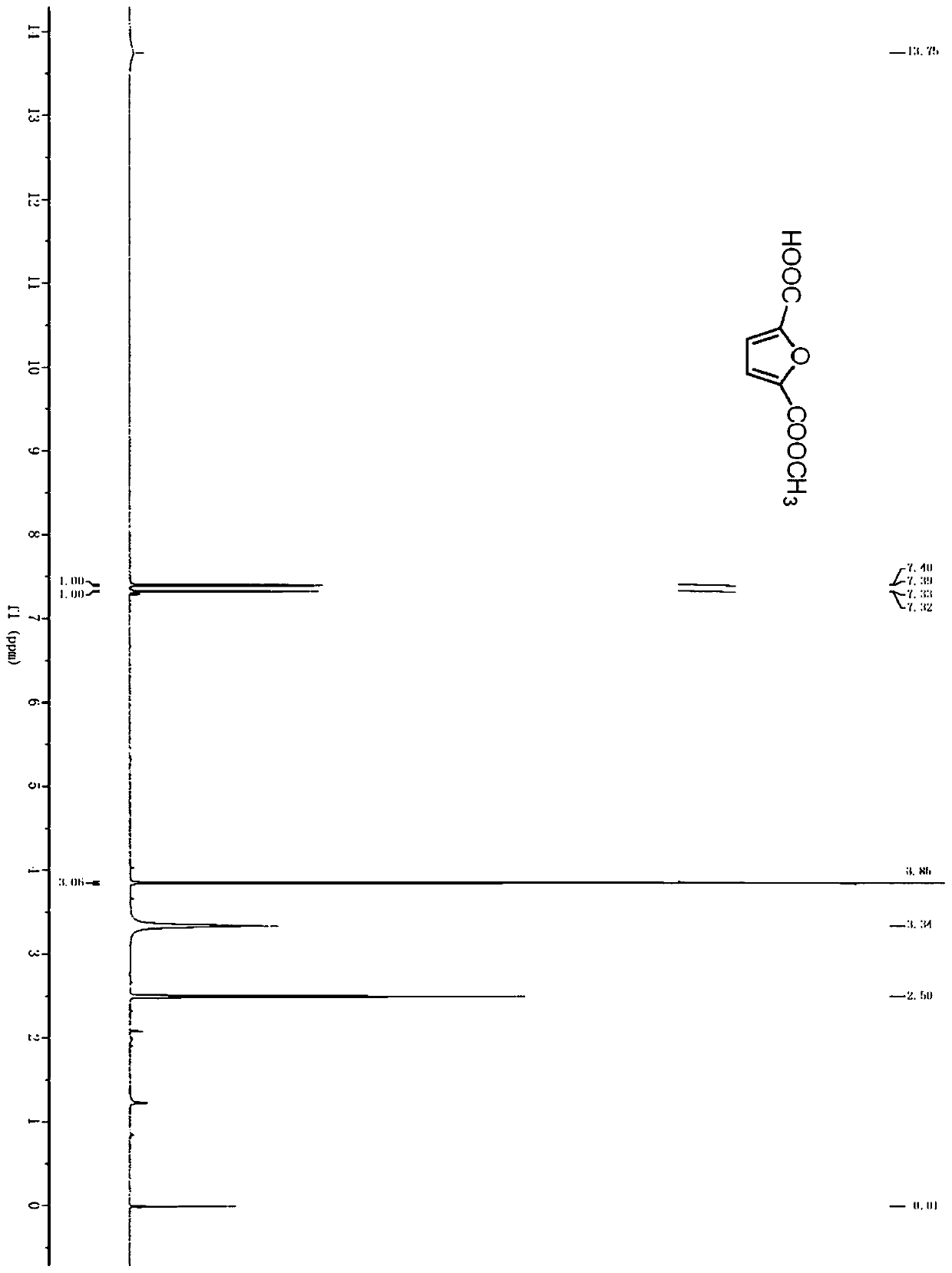
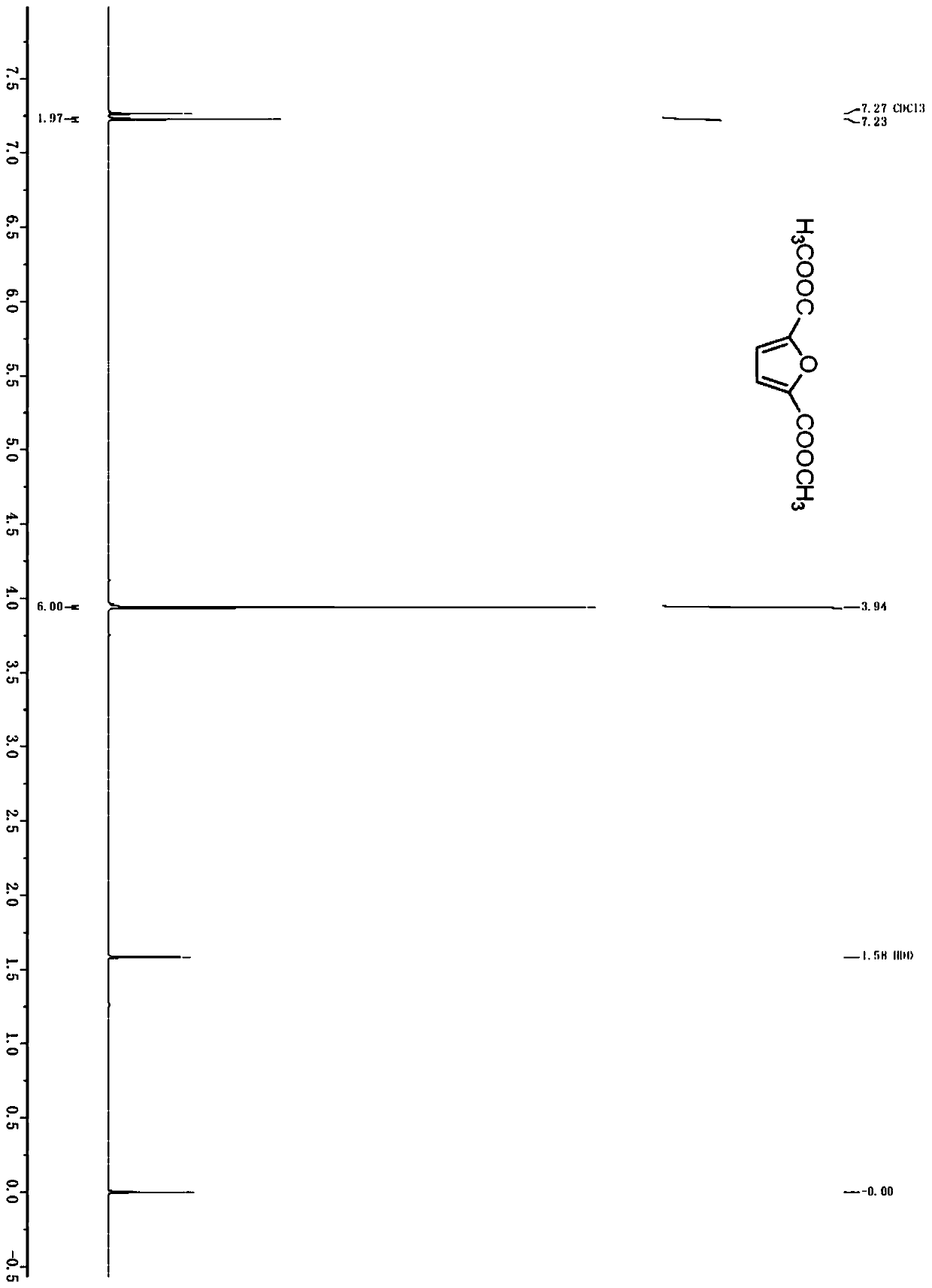
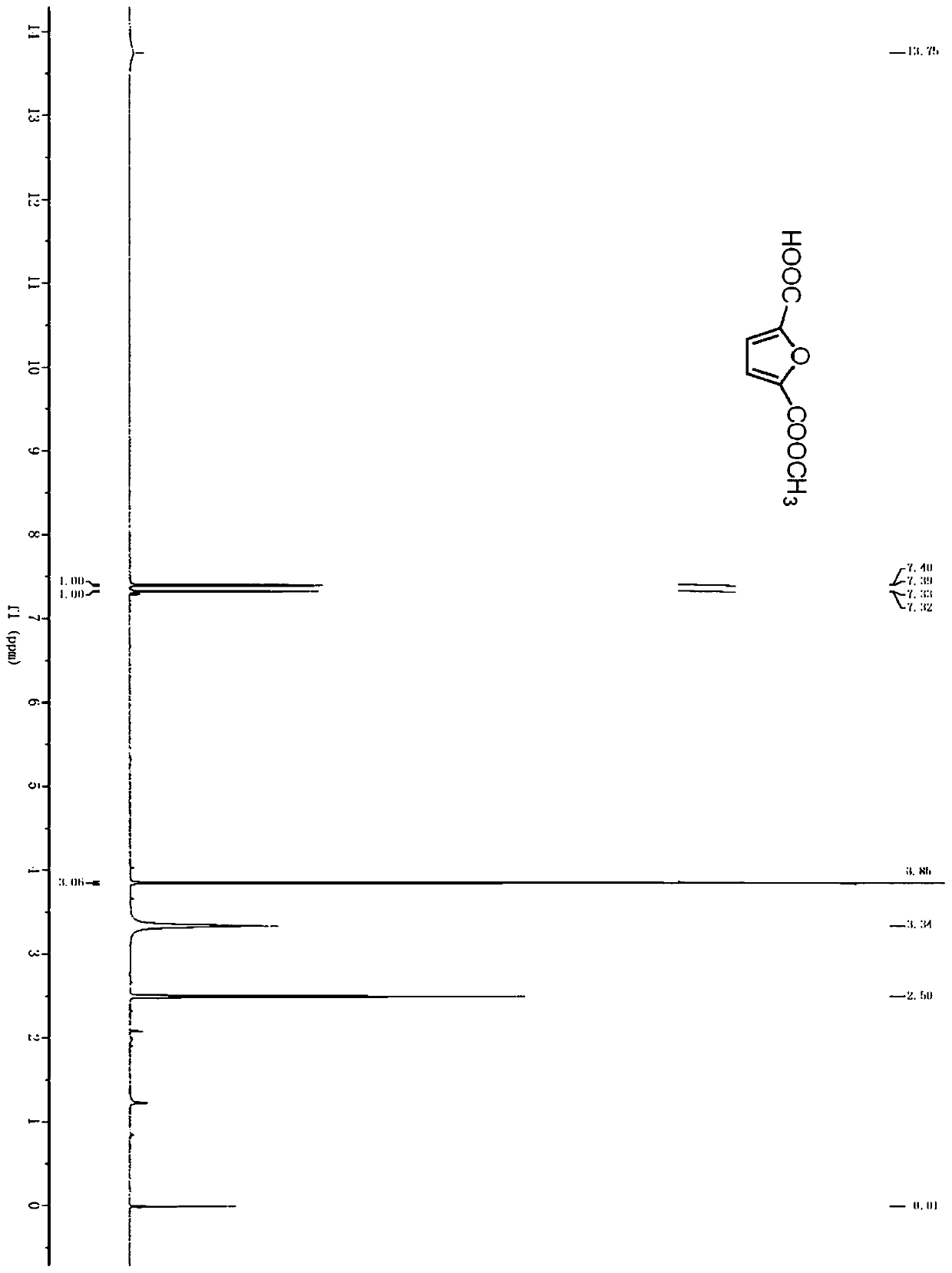
Method for producing furandicarboxylic acid and derivatives thereof from furfural
A technology of furandicarboxylic acid and furfural, applied in the direction of organic chemistry and the like, can solve the problems of high cost, difficult to have recyclability and catalytic stability, harsh reaction conditions, etc., and achieve the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of 2-methylfuran from furfural
[0036] Hydrogenation catalysts are prepared by impregnation. Pd precursor PdCl 2 Dissolved in a certain amount of water. A certain amount of SiO 2 Add PdCl 2 In solution, the metal occupies SiO 2 2% of mass. The mixture was stirred until dry, dried overnight at 80°C, and then ground into powder. The powder was calcined in a nitrogen atmosphere at 350°C, and then reduced with hydrogen at the same temperature to obtain a hydrogenation catalyst.
[0037] The present embodiment takes furfural as raw material. Add 1g of hydrogenation catalyst into the reaction kettle, add 10g of furfural, and then add 80mL of anhydrous methanol as a solvent. After sealing the reactor, first use N 2 Replace the air in the reactor, and then use H 2 Replace the N in the reactor 2 , then filled with 4MPa of H 2 . The temperature of the reaction kettle was raised to 150° C., and the reaction was carried out for 4 hours.
[0038] After...
Embodiment 2
[0054] (1) Preparation of 2-methylfuran from furfural
[0055] Hydrogenation catalysts are prepared by impregnation. Ni precursor Ni(NO 3 ) 2 ·6H 2 O dissolved in a certain amount of water. A certain amount of activated carbon is added to the precursor solution, and the metal accounts for 20% of the mass of the activated carbon. The mixture was stirred until dry, dried overnight at 80°C, and then ground into powder. The powder was calcined in a nitrogen atmosphere at 350°C, and then reduced with hydrogen at the same temperature to obtain a hydrogenation catalyst.
[0056] The present embodiment takes furfural as raw material. Add 1g of hydrogenation catalyst into the reaction kettle, add 10g of furfural, and then add 80mL of anhydrous tetrahydrofuran as a solvent. After sealing the reactor, first use N 2 Replace the air in the reactor, and then use H 2 Replace the N in the reactor 2 , then filled with 2MPa of H 2 . The reaction kettle was heated to 200°C and reacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com