Tri(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran carboxylate complex, and preparation method and application thereof
A furan formate, phenylpropyl technology, applied in tin organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve problems such as strong toxicity, application limitations, etc., and achieve low cost, simple preparation method, and anticancer activity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
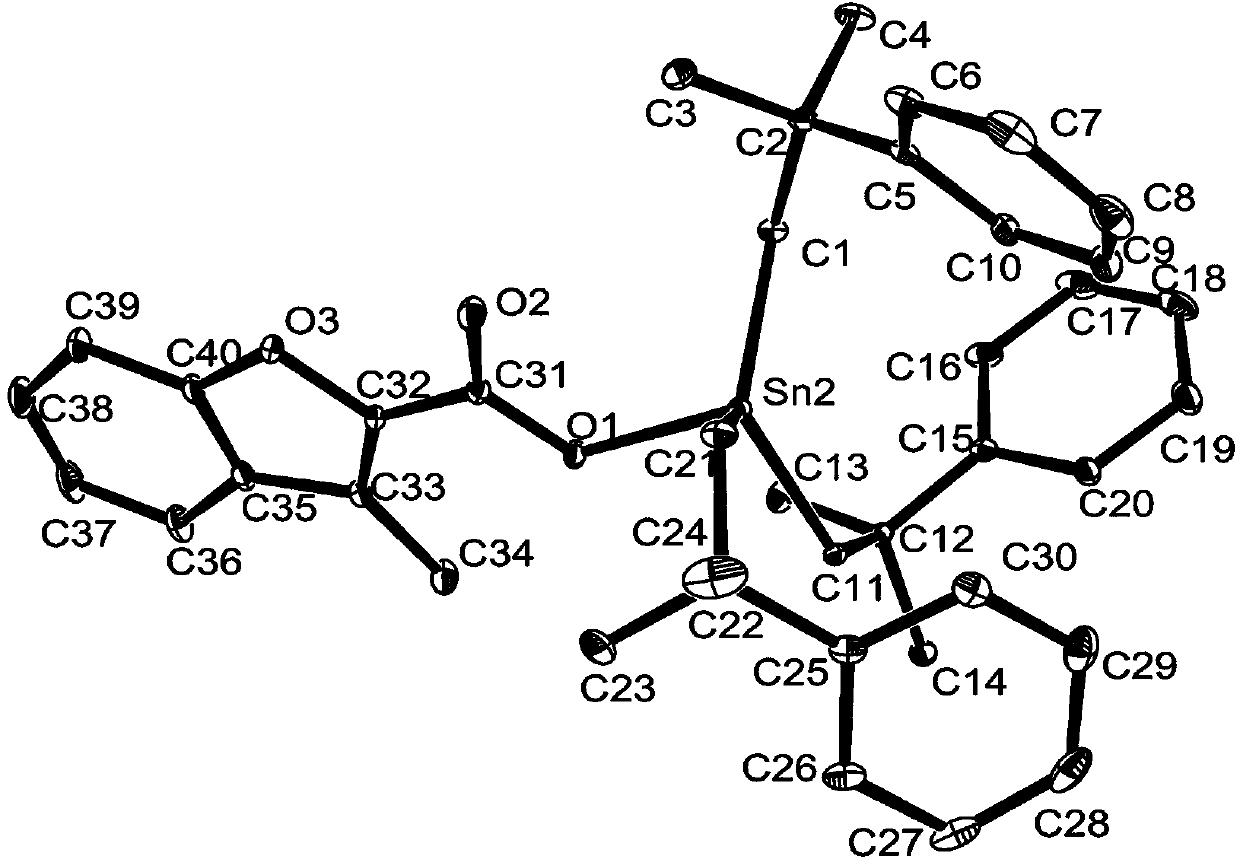
[0032] Preparation of three (2-methyl-2-phenylpropyl) tin 3-methylbenzofuranoate complexes:
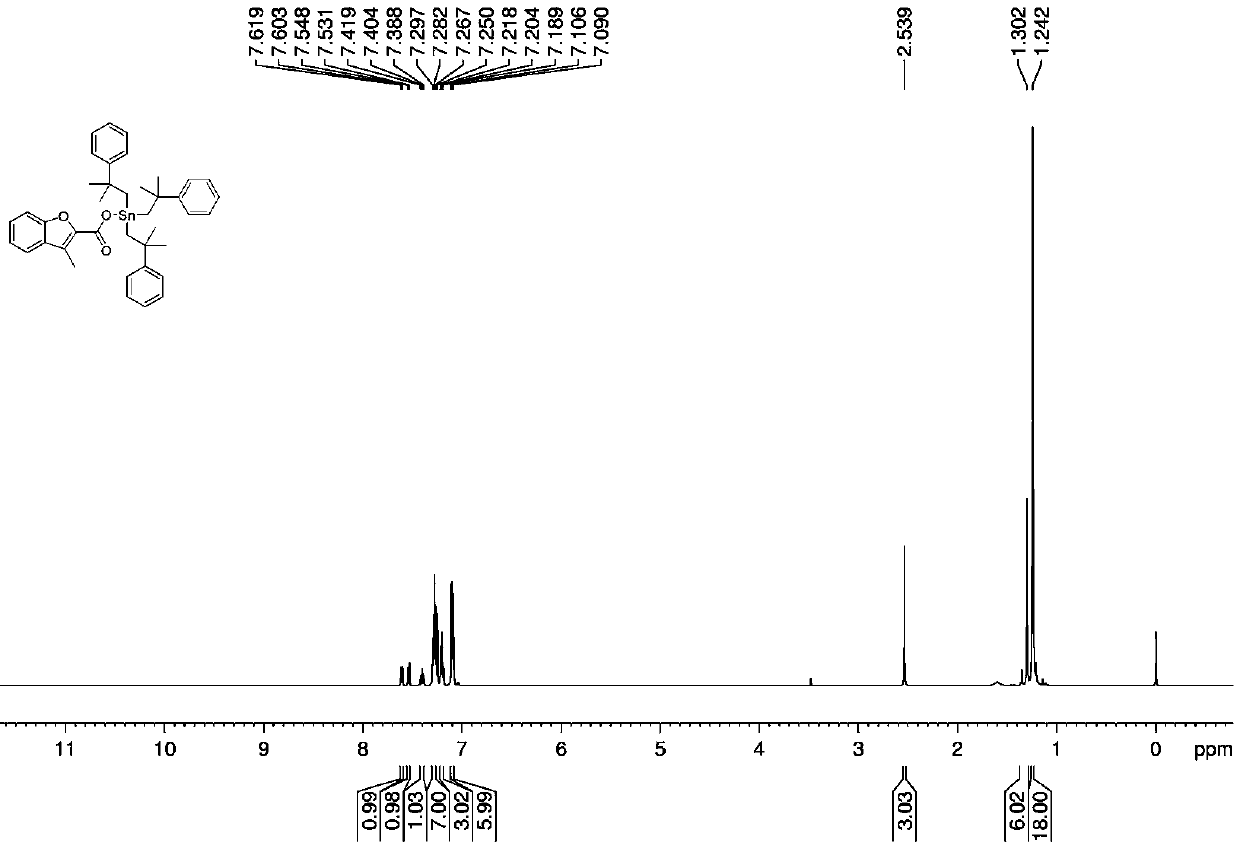
[0033] In a 250 mL round bottom flask, 1.0531 g (1.0 mmol) of bis[tris(2-methyl-2-phenylpropyl)]tin oxide and 0.3528 g of 3-methylbenzofurancarboxylic acid ( 2.0 mmol), solvent toluene 25 mL, install a Dean-Stark trap, and heat at reflux at 112-120 °C for 6 h. After the reaction, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a white solid, which is recrystallized from ethanol, namely tris(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran ester complexes. Yield: 70%, melting point: 112-114°C.
[0034] Elemental analysis (C 40 h 46 o 3 Sn): Theoretical: C, 69.28; H, 6.69. Found: C, 69.32; H, 6.65.
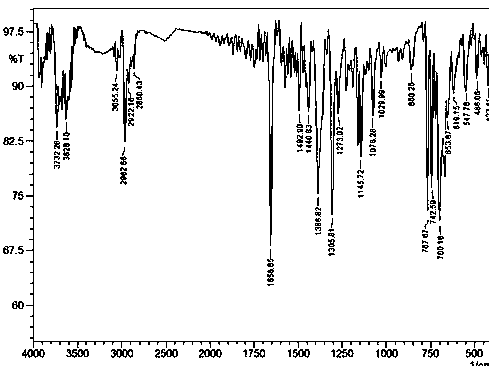
[0035] IR (KBr, v / cm -1 ): 3722.26 (m), 3628.10 (m), 3055.24 (m), 2962.66 (m), 2922.16 (m), 2860.43 (m), 1656.85 (s), 1492.90 (m), 1440.83 (m), 1386.82 (s ), 1305.81 (s), 1273.02 (㎜), 1145.72 (m), 1076.28 (m), 1029.99 (w), 860.25 (w...
Embodiment 2
[0041] Preparation of three (2-methyl-2-phenylpropyl) tin 3-methylbenzofuranoate complexes:
[0042] In a 250 mL round bottom flask, add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0540 g (1.0 mmol), 3-methylbenzofurancarboxylic acid 0.3880 g (2.2 mmol), solvent toluene 25 mL, install a Dean-Stark trap, and heat at reflux at 112-120 °C for 8 h. After the reaction is completed, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a white solid, which is recrystallized from ethanol, which is tris(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran ester complexes. Yield: 70%, melting point: 112-114°C.
[0043] Elemental analysis (C 40 h 46 o 3 Sn): Theoretical: C, 69.28; H, 6.69. Found: C, 69.32; H, 6.65.
[0044] IR (KBr, v / cm -1 ): 3722.26 (m), 3628.10 (m), 3055.24 (m), 2962.66 (m), 2922.16 (m), 2860.43 (m), 1656.85 (s), 1492.90 (m), 1440.83 (m), 1386.82 (s ), 1305.81 (s), 1273.02 (㎜), 1145.72 (m), 1076.28 (m), 1029.99 (w), ...
Embodiment 3
[0050] Preparation of three (2-methyl-2-phenylpropyl) tin 3-methylbenzofuranoate complexes:
[0051] In a 250 mL round bottom flask, add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0534 g (1.0 mmol), 3-methylbenzofurancarboxylic acid 0.3885 g (2.2 mmol), 35 mL of solvent toluene, installed a Dean-Stark trap, and heated to reflux at 112-120 °C for 8 h. After the reaction is completed, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a yellow solid, which is recrystallized from ethanol, namely tris(2-methyl-2-phenylpropyl)tin 3-methylbenzofuran ester complexes. Yield: 70%, melting point: 112-114°C.
[0052] Elemental analysis (C 40 h 46 o 3 Sn): theoretical value: C, 69.28; H, 6.69. Found: C, 69.32; H, 6.65.
[0053] IR (KBr, v / cm -1): 3722.26 (m), 3628.10 (m), 3055.24 (m), 2962.66 (m), 2922.16 (m), 2860.43 (m), 1656.85 (s), 1492.90 (m), 1440.83 (m), 1386.82 (s ), 1305.81 (s), 1273.02 (㎜), 1145.72 (m), 1076.28 (m), 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com