Bio-based benzoxazine resin containing furanamide structure and preparation method of bio-based benzoxazine resin
A furanamide and benzoxazine technology is applied in the field of bio-based furanamide structure-containing benzoxazine resin and its preparation, and can solve the problems that thermal properties need to be further improved, daidzein and resveratrol are expensive, and the like, Achieve the effects of low cost, high heat resistance, increased crosslink density and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
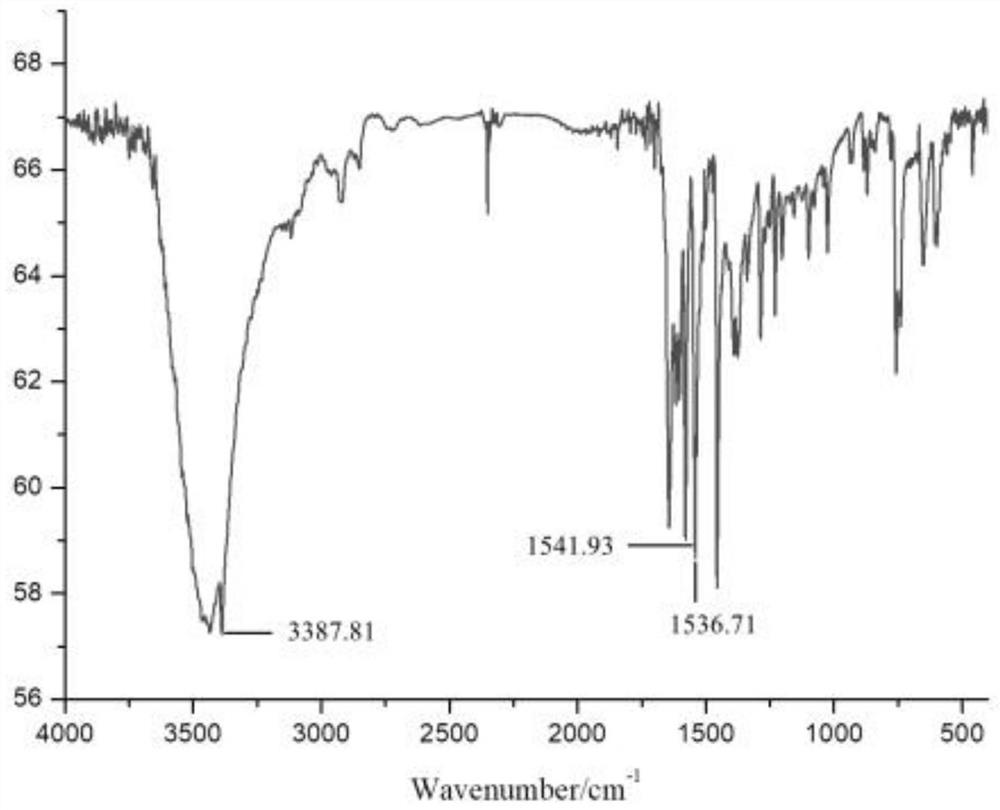
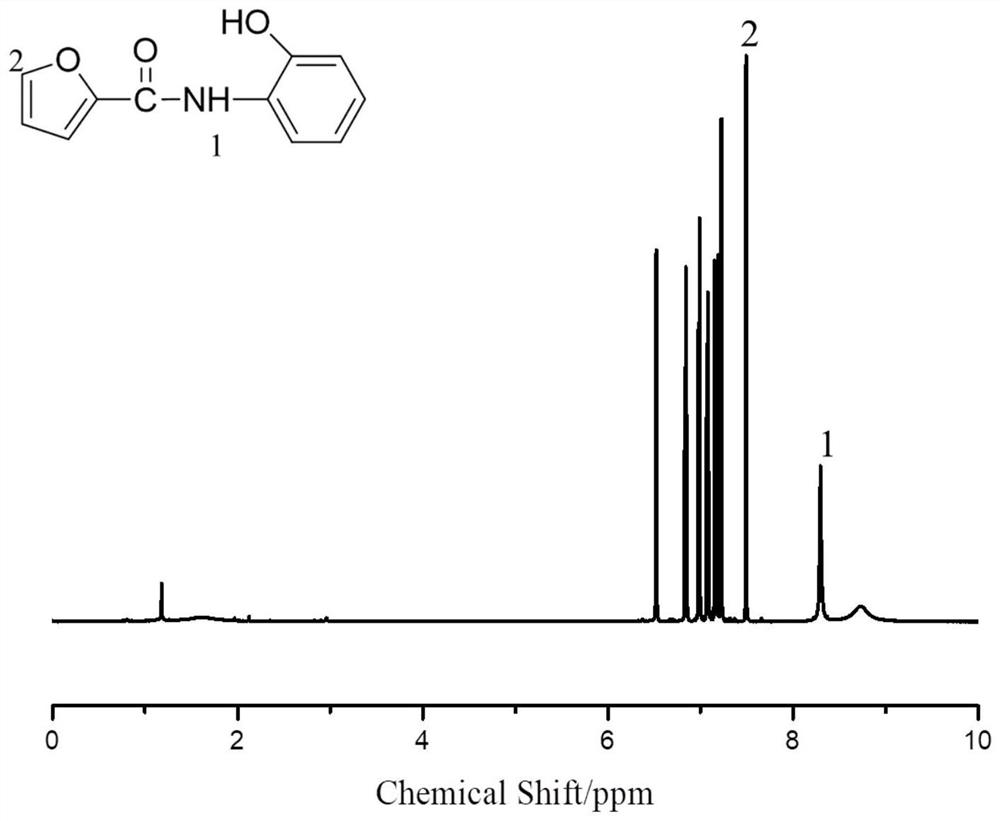
[0042] (1) Preparation of o-furamide structure phenol:
[0043] Add 21.826g (0.2mol) of o-aminophenol and 250mL of DMF into a 500mL three-necked flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer, then place it in an ice-water bath, stir and dissolve until completely transparent, and keep the system temperature at 0°C At ~5°C, start to add 28.72g (0.22mol) of furoyl chloride dropwise, keep the reaction temperature at 0°C~5°C for 24 hours, then add the reaction solution dropwise into ice water to precipitate, filter, and wash the filter cake with water After the filtrate is neutral, the filter cake is dried under vacuum conditions to finally obtain light gray o-furamide structure phenol with a yield of 94.1%.
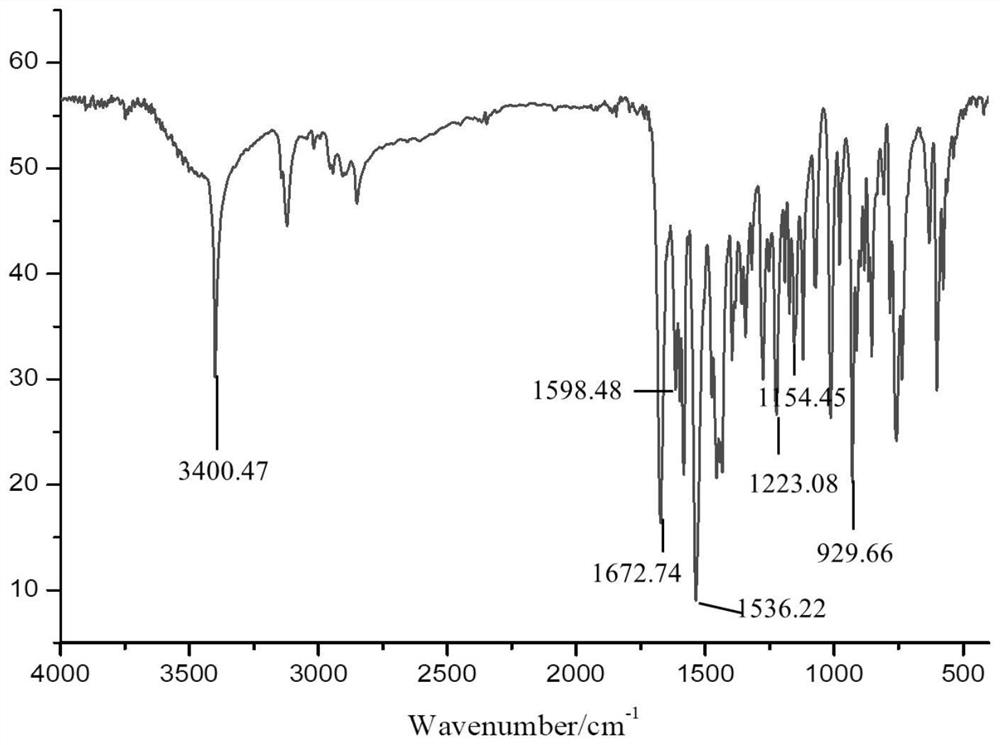
[0044] (2) Preparation of o-furamide structure phenol-aniline type benzoxazine:
[0045] In a three-neck flask equipped with a mechanical stirrer, a condenser, and a thermometer, sequentially add 20.32 (0.1mol) of o-furamide ...
Embodiment 2
[0047] (1) Preparation of p-furamide structure phenol:
[0048] Add 21.826g (0.2mol) of p-aminophenol and 250mL of DMAc into a 500mL three-neck flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer, then place it in an ice-water bath, stir and dissolve until completely transparent, and keep the system temperature at 0°C At ~5°C, start to add 28.72g (0.22mol) of furoyl chloride dropwise, keep the reaction temperature at 0°C~5°C for 24 hours, then add the reaction solution dropwise into ice water to precipitate, filter, and wash the filter cake with water Until the filtrate is neutral, the filter cake is dried under vacuum conditions to finally obtain off-white p-furamide structure phenol with a yield of 93.4%.
[0049] (2) Preparation of o-furamide structure phenol-furfuryl amine type benzoxazine:
[0050] In a three-neck flask equipped with a mechanical stirrer, a condenser, and a thermometer, sequentially add 20.32 (0.1mol) of o-fura...
Embodiment 3
[0052] (1) Preparation of o-furamide structure phenol:
[0053] Add 21.826g (0.2mol) of o-aminophenol and 250mL of DMF into a 500mL three-necked flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer, then place it in an ice-water bath, stir and dissolve until completely transparent, and keep the system temperature at 0°C At ~5°C, start to add 28.72g (0.22mol) of furoyl chloride dropwise, keep the reaction temperature at 0°C~5°C for 24 hours, then add the reaction solution dropwise into ice water to precipitate, filter, and wash the filter cake with water After the filtrate is neutral, the filter cake is dried under vacuum conditions to finally obtain light gray o-furamide structure phenol with a yield of 94.1%.
[0054] (2) Preparation of o-furamide structure phenol-4,4'-diaminodiphenylmethane type benzoxazine:
[0055] In a three-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, sequentially add 20.32 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com