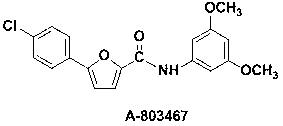
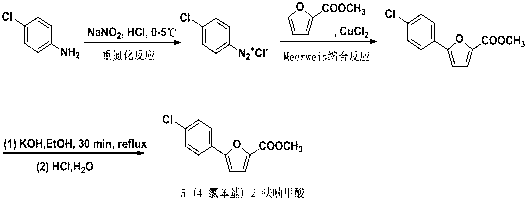
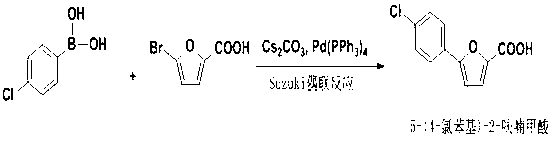Synthetic method of A-803467 key intermediate
An intermediate and key technology, applied in the field of organic synthesis, can solve the problems of low atom utilization rate, use of toxic and harmful raw materials, harsh reaction conditions, etc., and achieve extremely easy control of reaction conditions, improve the quality of diazonium salts, and simplify the reaction operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 4.6g (0.041mol) of furanic acid, 12.76g (0.1mol) of p-chloroaniline, 40ml of acetone, 3.5g of CuCl in the three-necked flask 2 2H 240ml of aqueous solution of O, stirred, and slowly dripped 5ml of tert-butyl nitrite into the system with a constant pressure funnel, and controlled the rate of addition so that the temperature of the system did not exceed 10°C. : ethanol: sherwood oil: 4: 1) tracking to complete reaction. There is red insoluble matter in the system, open the door overnight, filter with suction, extract the filtrate under reduced pressure for 20 minutes, then extract with ethyl acetate, take the upper organic phase, and dissolve the obtained red filter cake in the organic phase, use Extract with an appropriate amount of saturated sodium bicarbonate, separate liquids while hot, wash the aqueous phase with 10% hydrochloric acid aqueous solution until acidic, a large amount of white solid appears in the system, filter with suction, dry to a constant weight...
Embodiment 2
[0037] Add 2.3g (0.020mol) of furanic acid, 12.76g (0.1mol) of p-chloroaniline, 40ml of ethanol, 1.75g of CuCl in a three-necked flask 2 2H 2 40ml of aqueous solution of O, stir, slowly drop 5ml of isopropyl nitrite solution into the system with a constant pressure funnel, control the rate of addition so that the system temperature does not exceed 20°C, after the addition is completed, keep the reaction for 5h, TLC (developing agent: Ethanol:petroleum ether: 4:1) track until the reaction is complete. There is red insoluble matter in the system, open the door overnight, filter with suction, extract the filtrate under reduced pressure for 20 minutes, then extract with ethyl acetate, take the upper organic phase, and dissolve the obtained red filter cake in the organic phase, use Extract with an appropriate amount of saturated sodium bicarbonate, separate liquids while hot, wash the aqueous phase with 10% hydrochloric acid aqueous solution until acidic, a large amount of white...
Embodiment 3
[0039] In a 250ml three-necked flask, add 7.8g (0.07mol) of furanic acid, 12.76g (0.1mol) of p-chloroaniline, 50ml of acetone, 6g of CuBr 2 40ml of aqueous solution, stirred, and slowly dripped 10ml of tert-butyl nitrite into the system with a constant pressure funnel, and controlled the rate of addition so that the system temperature did not exceed 30°C. Ethanol:petroleum ether: 4:1) track until the reaction is complete. There is red insoluble matter in the system, open the door overnight, filter with suction, extract the filtrate under reduced pressure for 20 minutes, then extract with ethyl acetate, take the upper organic phase, and dissolve the obtained red filter cake in the organic phase, use Extract with an appropriate amount of saturated sodium bicarbonate, separate liquids while hot, wash the aqueous phase with 10% hydrochloric acid aqueous solution until acidic, a large amount of white solid appears in the system, filter with suction, dry to a constant weight of 12.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com