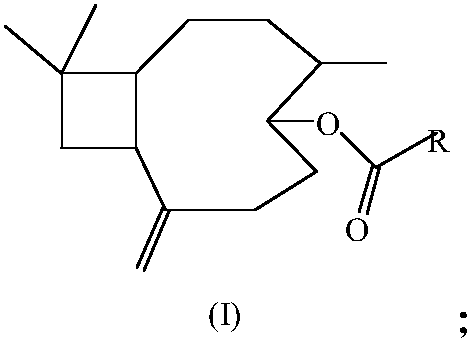
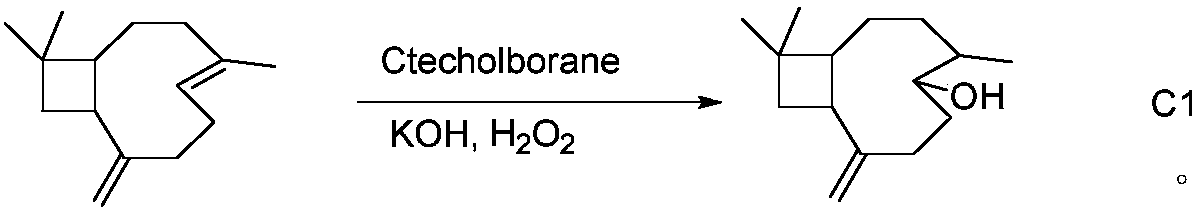Furoic acid beta-caryophyllene-5-ester compound as well as preparation method and application of furoic acid beta-caryophyllene-5-ester compound
A technology of ester compounds and furan formic acid, applied in organic chemistry, drug combination, antitumor drugs, etc., can solve the problem that β-caryophyllene derivatives do not have much breakthrough progress, and the biological activity research of β-caryophyllene derivatives There are no outstanding reports and other problems, and the effects of good inhibitory activity, simple reaction steps and good application prospects are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis method of β-caryophyllene alcohol C1 (6,10,10-trimethyl-2-methylenebicyclo[7.2.0]undecan-5-ol):
[0032] Add 4.4mmol of β-caryophyllene to 6.6mL of 1M solution of catecholborane in tetrahydrofuran, reflux at 80°C for 18h, and use 40mL of CH 2 Cl 2 Dilute and cool, add 20mL 3M KOH and 20mL 30% H 2 o 2 React for 30 minutes, wash with saturated NaCl for 3 times, dry, and remove the solvent to obtain a dark yellow oily liquid, which is eluted with a 100-200 mesh silica gel column, mobile phase petroleum ether: ethyl acetate = 1: 7, and a light yellow oily liquid C1 is obtained . The reaction process is as follows:
[0033]
[0034] C1 1 HNMR (600M, DMSO-d 6 ) δ: 4.83 (d, 2H, =CH 2 , J=6Hz), 4.22(d, 1H, -OH, J=6Hz), 3.32(s, 1H, -CH), 2.46-2.42(m, 1H, -CH), 2.22-2.12(m, 2H, -CH 2 ), 1.92-1.86 (m, 1H, -CH), 1.74-1.71 (m, 2H, -CH 2 ), 1.70 (t, 1H, -CH, J=6Hz), 1.58-1.53 (m, 1H, -CH), 1.51-1.48 (m, 2H, -CH 2 ), 1.44-1.39 (m, 2H, -CH 2 ), 0.38(s, 3H...
Embodiment 2
[0041] Synthetic method of furan-3-carboxylic acid β-caryophyllene-5-ester C3 (6,10,10-trimethyl-2-methylenebicyclo[7.2.0]undecan-5-ylfuran-3-carboxylate):
[0042] Dissolve furan-3-carboxylic acid 0.9 mmol and DCC 0.9 mmol in 5 mL of CH 2 Cl 2 , react at 0°C for 30min; add C10.9mmol prepared in Example 1 and dissolve in 1mL CH 2 Cl 2 DMAP 0.05mmol, reacted at room temperature for 5h, washed, dried, and solvent removed; eluted through a silica gel column to obtain 145mg of yellow oily liquid C3. The reaction equation is as follows:
[0043]
[0044] C3 1 HNMR (600M, CDCl 3 )δ: 8.00 (d, 1H, -CH, J = 6Hz), 7.41 (d, 1H, -CH, J = 1.2Hz), 6.75-6.73 (m, 1H, -CH), 4.97-4.90 (m, 2H, =CH2), 2.52-2.42(m, 1H, CH), 2.33-2.30(m, 1H, CH), 2.13-2.10(m, 1H, CH), 2.03-1.98(m, 1H, CH), 1.86-1.83(m, 1H, CH), 1.79-1.76(m, 2H, CH2), 1.66-1.63(m, 2H, CH2), 1.621.60(m, 2H, CH2), 1.42(s, 1H, CH), 1.26(d, 1H, CH, J=18Hz), 1.02(s, 3H, CH3), 0.99(s, 3H, CH3), 0.96(d, 1H, CH, J=12Hz), 0.90-0.8...
Embodiment 3
[0046] 1. NO inhibition rate experiment of compounds C2 and C3:
[0047] (1) Take the mouse macrophage RAW264.7 with a logarithmic growth cycle, seed it in a 96-well plate at 30,000 to 40,000 per well, at 37°C, 5% CO 2 Incubate in an incubator for 24 hours; take out the culture plate, remove the medium, and wash with PBS for 3 to 4 times;
[0048] (2) Set the control group, LPS+dexamethasone (DIM) positive drug group and compound C2, C3 sample groups; the experimental groups are as follows:
[0049] Control group: 1. Add 50 μL of 2 μg / mL LPS and 50 μL of caryophyllene CO at concentrations of 40, 20, 10, 5, and 2.5 μM to each well; 2. Add 50 μL of 2 μg / mL LPS and 50 μL of LPS to each well β-caryophyllenol C1 at 40, 20, 10, 5, 2.5 μM, respectively;
[0050] LPS+DIM positive drug group: Add 50 μL of 2 μg / mL LPS and 50 μL of DIM with concentrations of 40, 20, 10, 5 and 2.5 μM to each well;
[0051] Compound C2 sample group: add 50 μL of 2 μg / mL LPS and 50 μL of C2 at concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com