Methods and systems to form propylene chlorohydrin and propylene oxide
A technology of chloropropanol and chloride, applied in the field of forming PCH, which can solve the problems such as the difficulty in realizing the economical production process of propylene oxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
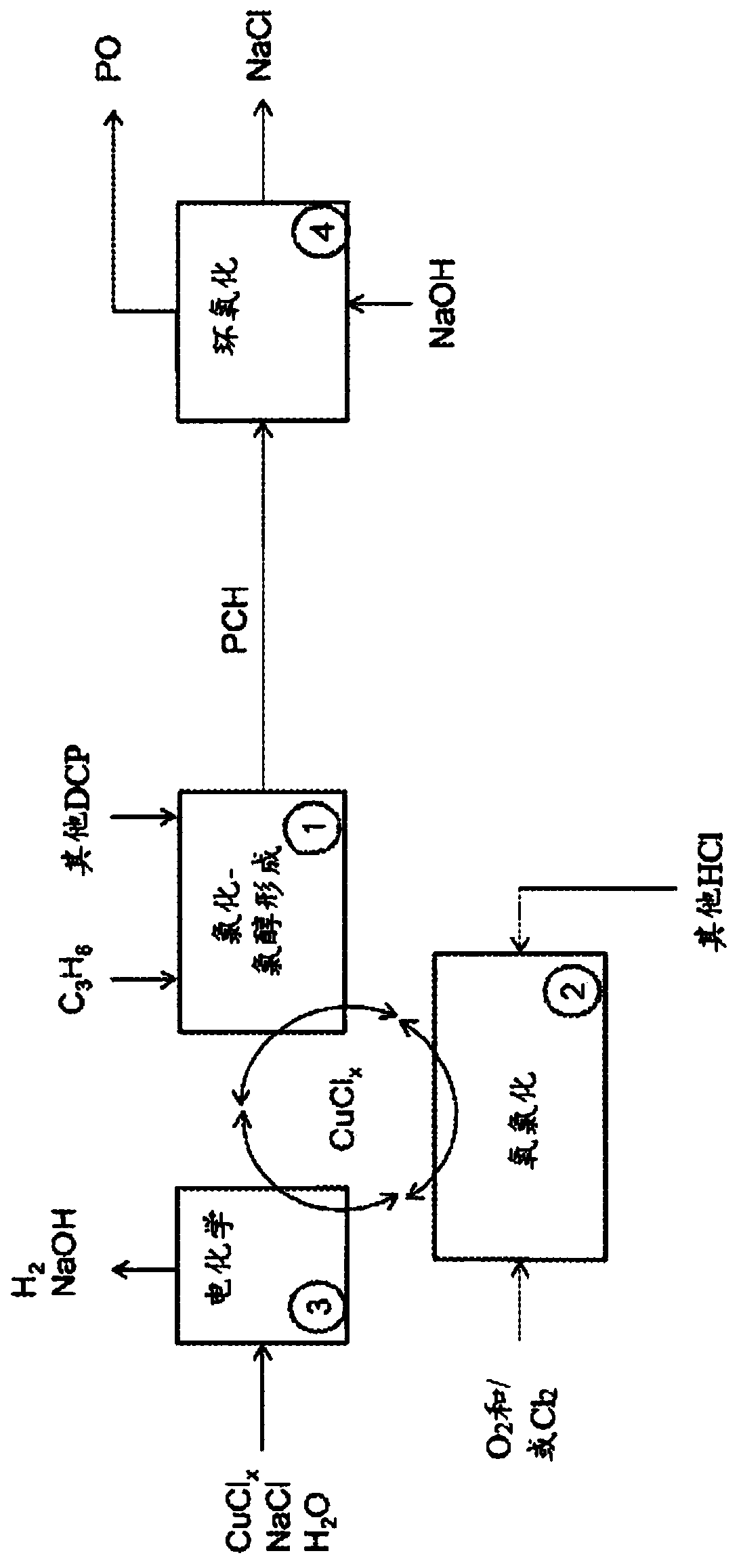
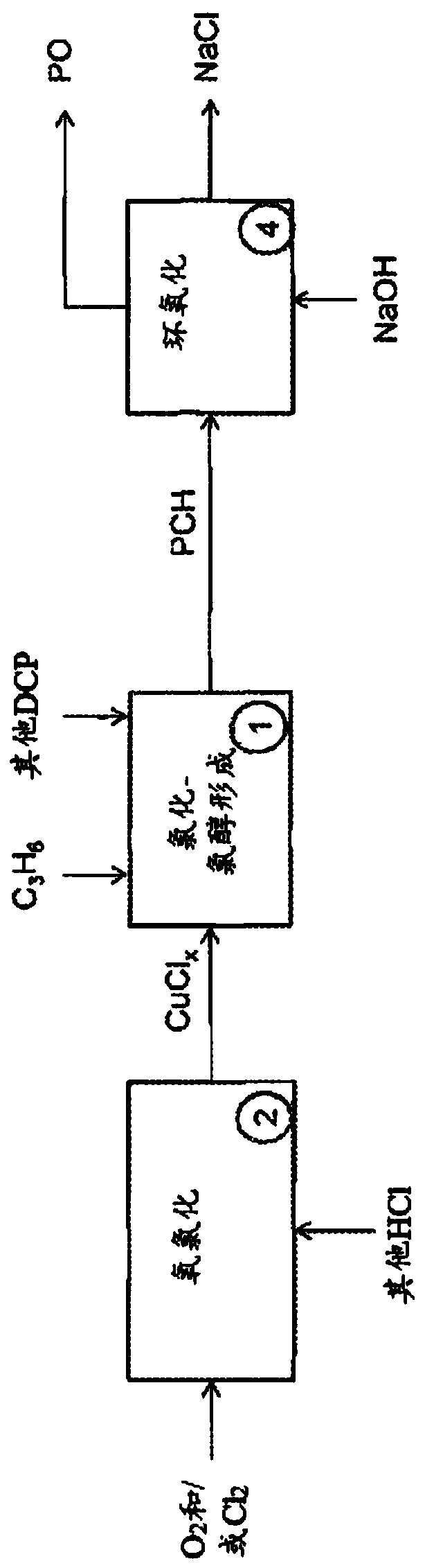
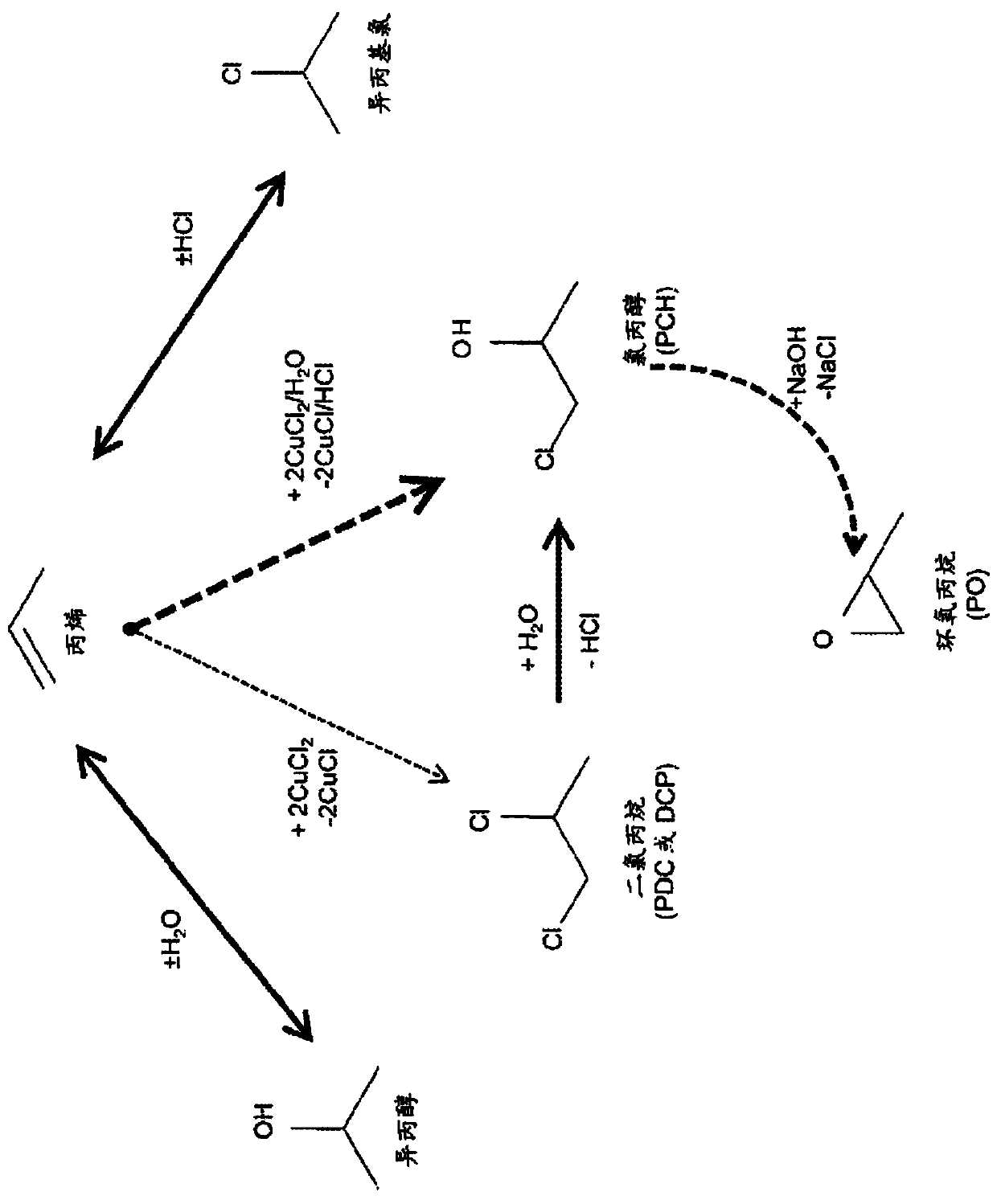
[0216] Use copper chloride to form PCH, DCP, isopropanol and isopropyl chloride from propylene
[0217] Experiment 1: In a Parr reactor under propylene pressure, CuCl 2 (1.0 mol / kg), CuCl (0.19 mol / kg), NaCl (0.66 mol / kg) and HCl (0.0091 mol / kg) solution were heated to 130°C for 15 minutes. The reactor was depressurized at 0°C into a bubble trap to trap volatile compounds. When the reactor is opened, the solution is extracted three times with an organic solvent (e.g., ethyl acetate or dichloromethane), and analyzed by a gas chromatography equipped with a mass spectrometer. A total of 11.4umol of DCP and 12.0umol of PCH were measured. The amount of recyclable products is 622umol isopropanol, 47.9umol acetone and 73.0umol isopropyl chloride.
[0218] Experiment 2: In a Parr reactor under propylene pressure, CuCl 2 (0.71mol / kg), CuCl (0.71mol / kg) and NaCl (2.76mol / kg) solutions are heated to 150°C for 15 minutes. The reactor was depressurized at 0°C into a bubble trap to trap volat...
Embodiment 2
[0220] Use palladium chloride and copper chloride to form PCH from propylene
[0221] In the Parr reactor under propylene pressure, CuCl 2 (2.80mol / kg), CuCl (0.54mol / kg), NaCl (1.84mol / kg) and PdCl 2 (0.012mol / kg) solution was heated to 130°C for 15 minutes. The reactor was depressurized at 0°C into a bubble trap to trap volatile compounds. When the reactor is opened, the solution is extracted three times with an organic solvent (e.g., ethyl acetate or dichloromethane), and analyzed by a gas chromatography equipped with a mass spectrometer. A total of 0.29 mmol of DCP and 1.24 mmol of PCH were measured. The measured amount of recyclable product was 9.23 mmol of isopropanol, 3.17 mmol of acetone and 10.9 mmol of isopropyl chloride.
Embodiment 3
[0223] Recycling of isopropanol
[0224] Put CuCl in Parr reactor 2 (3.0 mol / kg), CuCl (0.50 mol / kg) and NaCl (2.0 mol / kg) solutions were heated to 140°C for 15 minutes with the addition of isopropanol. At 0℃, keep using N 2 The flow sweeps the reactor into a bubble trap to trap volatile compounds. When the reactor is opened, the solution is extracted three times with an organic solvent (e.g., ethyl acetate or dichloromethane), and analyzed by a gas chromatography equipped with a mass spectrometer. Propylene, isopropanol, isopropyl chloride, PCH and DCP were all detected, indicating that isopropanol can be recycled and converted into the desired product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com