Monovalent anti-properdin antibodies and antibody fragments
A technology of antibody fragments and monovalent antibodies, applied in the field of medicine for the treatment of diseases mediated by disorders of the alternative complement pathway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0282] Example 1.V HH - Generation of His fusion cloning (in-fusion cloning) vector
[0283] The pBNJ391 vector was digested with restriction enzymes BstEII and EcoRI to remove the hinge and Fc. The vector was gel purified to yield a 1000 bp release product. The annealed oligonucleotides UDEC6629 / 6630 were cloned into pBNJ391 vector using BstEII / EcoRI. Annealed oligonucleotides contain the following sequences:
[0284] UDEC 6629 forward primer:
[0285] GTCACCGTGTCGAGCCATCATCACCATCATCATCACTGATGAG (SEQ ID NO: 65)
[0286] UDEC 6630 reverse primer:
[0287] AATTCTCATCATTTGTCATCATCATCCTTATAGTCGCTCGACACG (SEQ ID NO: 66)
[0288] The final vector contains BstEII-6xHis-EcoRI sites.
[0289] Next, the pNGH0320 vector was digested with XhoI / BstEII (yielding a 13 bp release product) and column purified. Next, use V HH Phage cloning templates for PCR amplification of inserts. Forward primer UDEC 6438 (GTCCACTCCCTCGAGGTGCAGCTGGTGGAGTCTGGG; SEQ ID NO: 67) and reverse primer UDEC...
Embodiment 2
[0304] Example 2. Anti-properdin V HH Antibody binding to human properdin
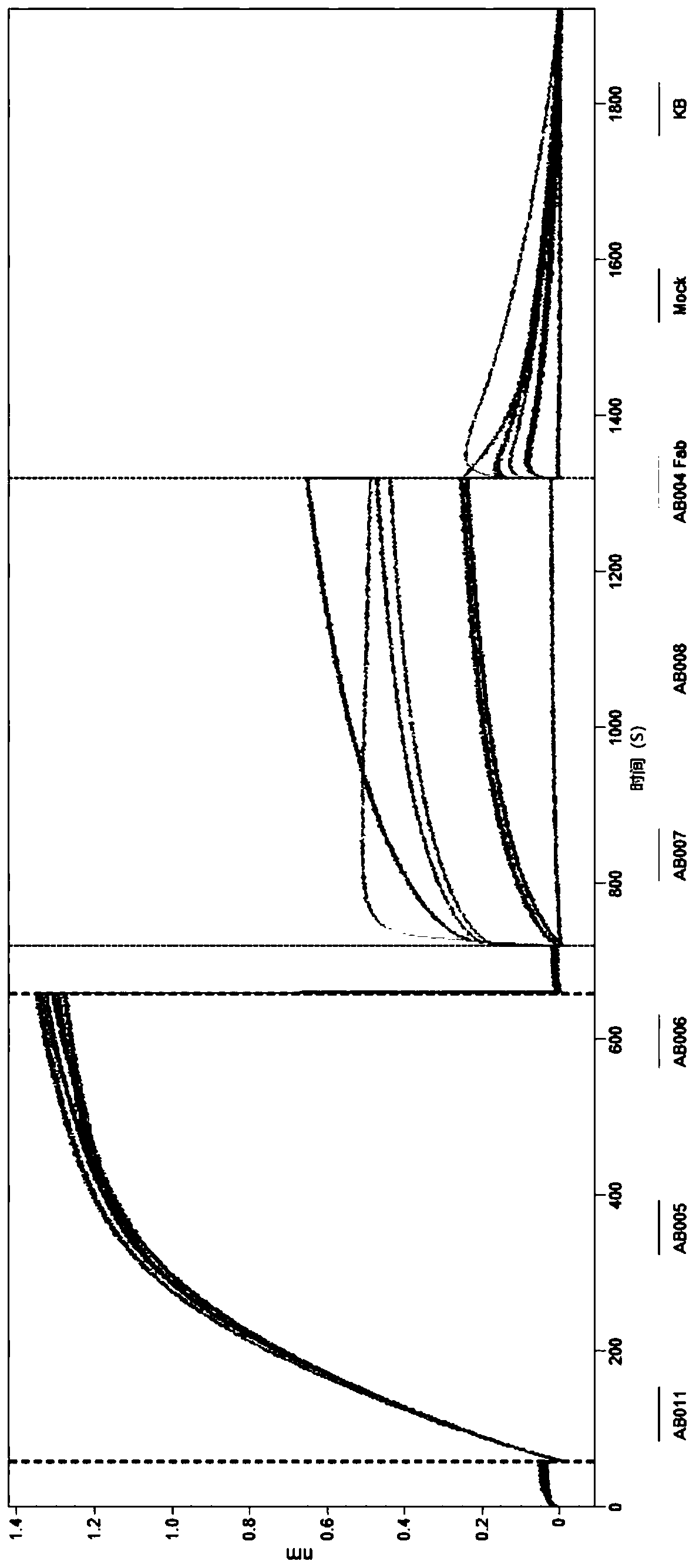
[0305] figure 1 Display kinetic binding measurements can be performed on an Octet instrument (FortéBio Inc.). All washes, dilutions and measurements were performed in kinetic buffer (FortéBio cat 185032) with plates shaking at 1000 rpm. Streptavidin biosensors (Forte Bio Cat: 18-5019 lot: 1405301 ) were equilibrated in kinetic buffer for 10 minutes before loading with 50nm biotinylated human properdin. For the binding phase, add 10 μg / mL of the anti-properdin antibody of choice or kinetic buffer blank, respectively, to the biosensor preloaded with biotinylated human properdin. The results show that AB005, AB006, AB007 and AB008 bind to human properdin.
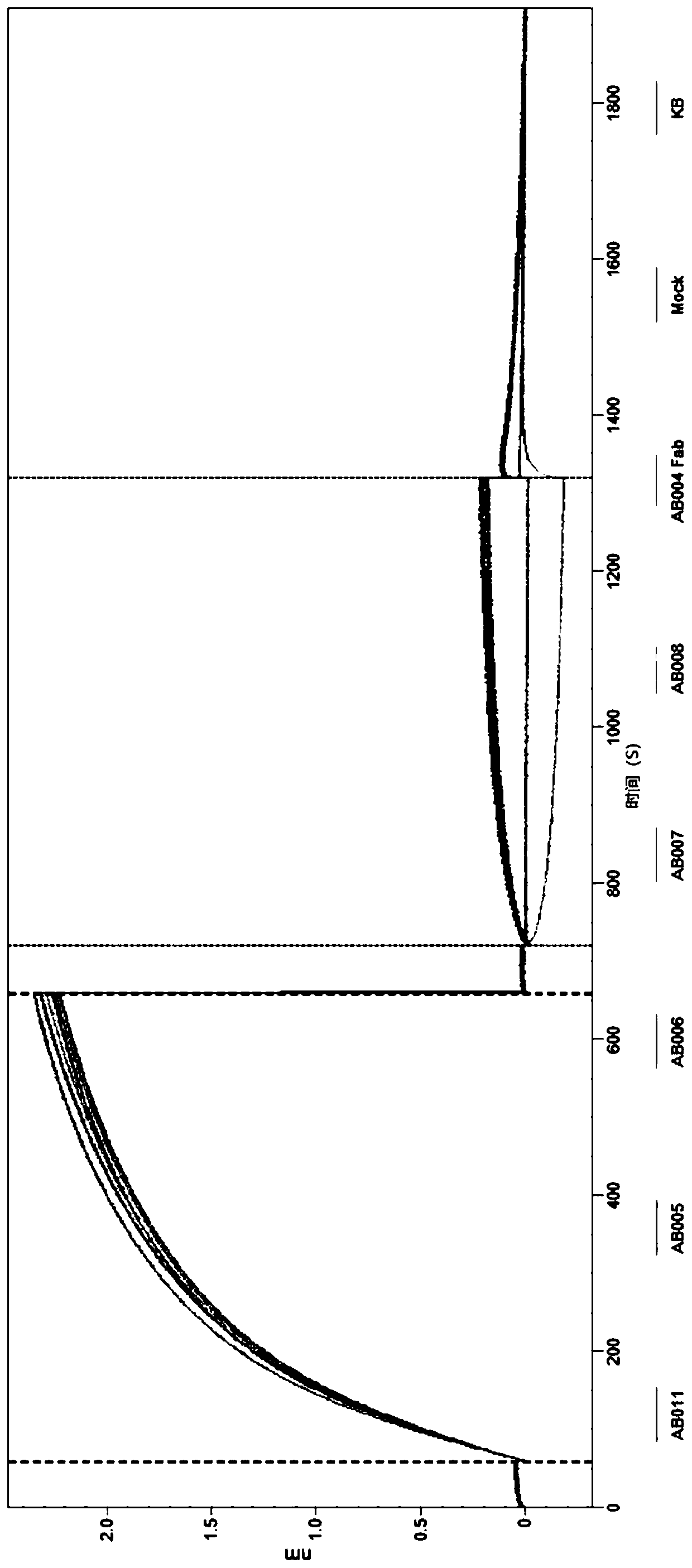
[0306] figure 2 Shows that kinetic binding measurements can be performed on an Octet instrument (Forté Bio Inc). All washes, dilutions and measurements were performed in kinetic buffer (Forté Biocat 185032) with plates shaking at 1000 rpm. Stre...
Embodiment 3
[0308] Example 3 Alternative Complement Hemolysis Assay
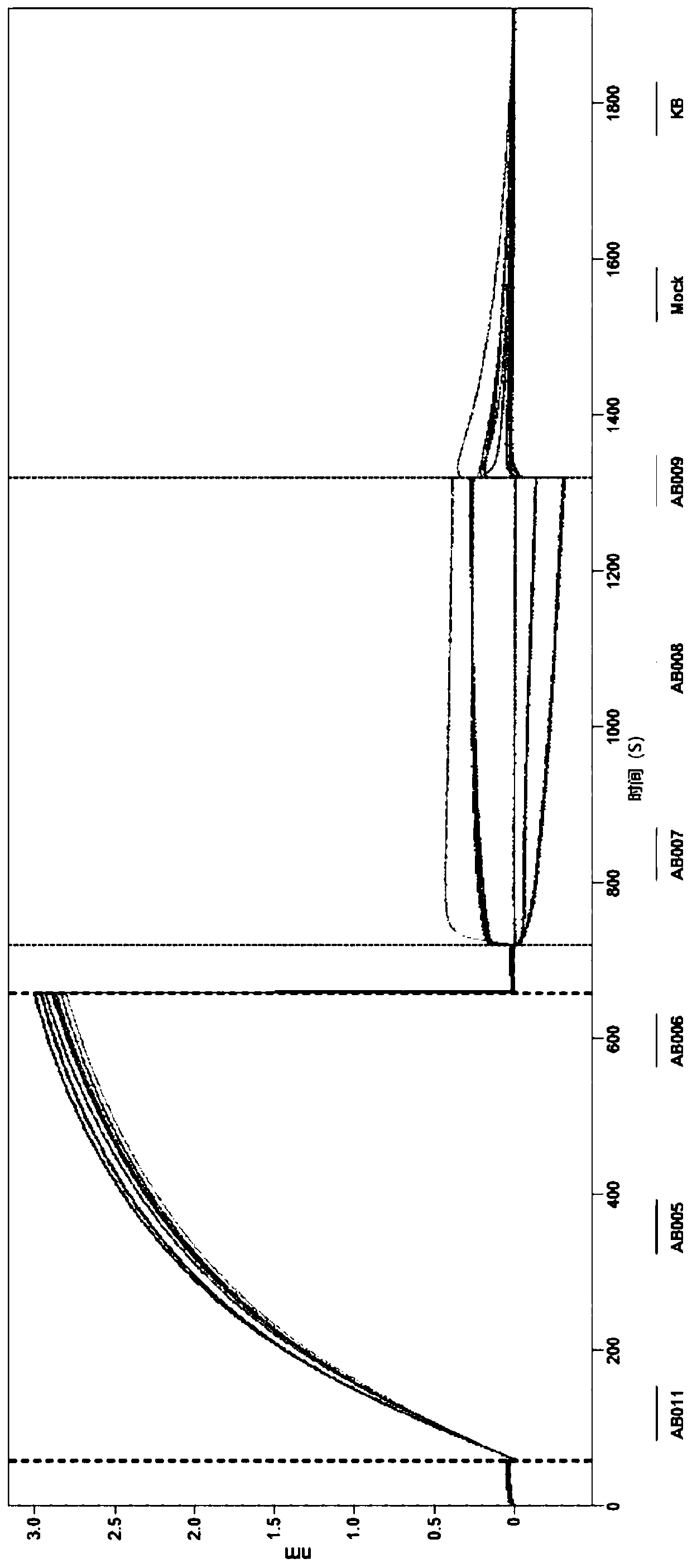
[0309] Figure 4 An alternative complement pathway mediated hemolysis assay based on the formation of terminal complement complexes on the surface of rabbit red blood cells (rRBC) is shown. Due to the formation of this complex, rRBCs are lysed. Agents that inhibit complement complex formation are expected to inhibit cell lysis. Various anti-properdin antigen-binding fragments were tested to assess effects on cell lysis mediated by alternative complement activation. by supplementing with 10 mM EGTA and 10 mM MgCl 2 Dilute 40% normal human serum in gelatin veronal buffer (GVB) (eg, 1600 μL normal human serum) to supplemented with 10 mM EGTA and 10 mM MgCl 2 "Assay plate" was prepared in 2400 μL of GVB). 50 μL of this solution was dispensed into each well of an assay plate (polystyrene). Next, by dissolving 50 μL / well of 2× mAb (eg, anti-properdin Fab) in supplemented with 10 mM EGTA and 0-10 mM MgCl 2 Dilution plat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap