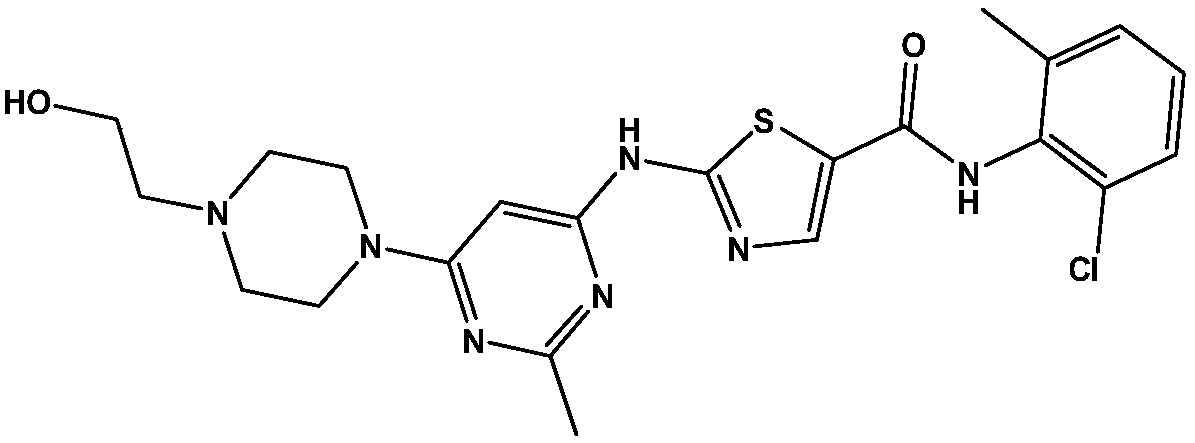
Dasatinib crystal form and preparation method thereof
A technology for dasatinib and Nijing, which is applied in the field of dasatinib crystal form and preparation, can solve the problems that the solubility of the crystal form needs to be improved, the preparation process is cumbersome, and the solubility of the crystal form is low, and the preparation method is simple and feasible, Excellent physical and chemical properties and stable product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
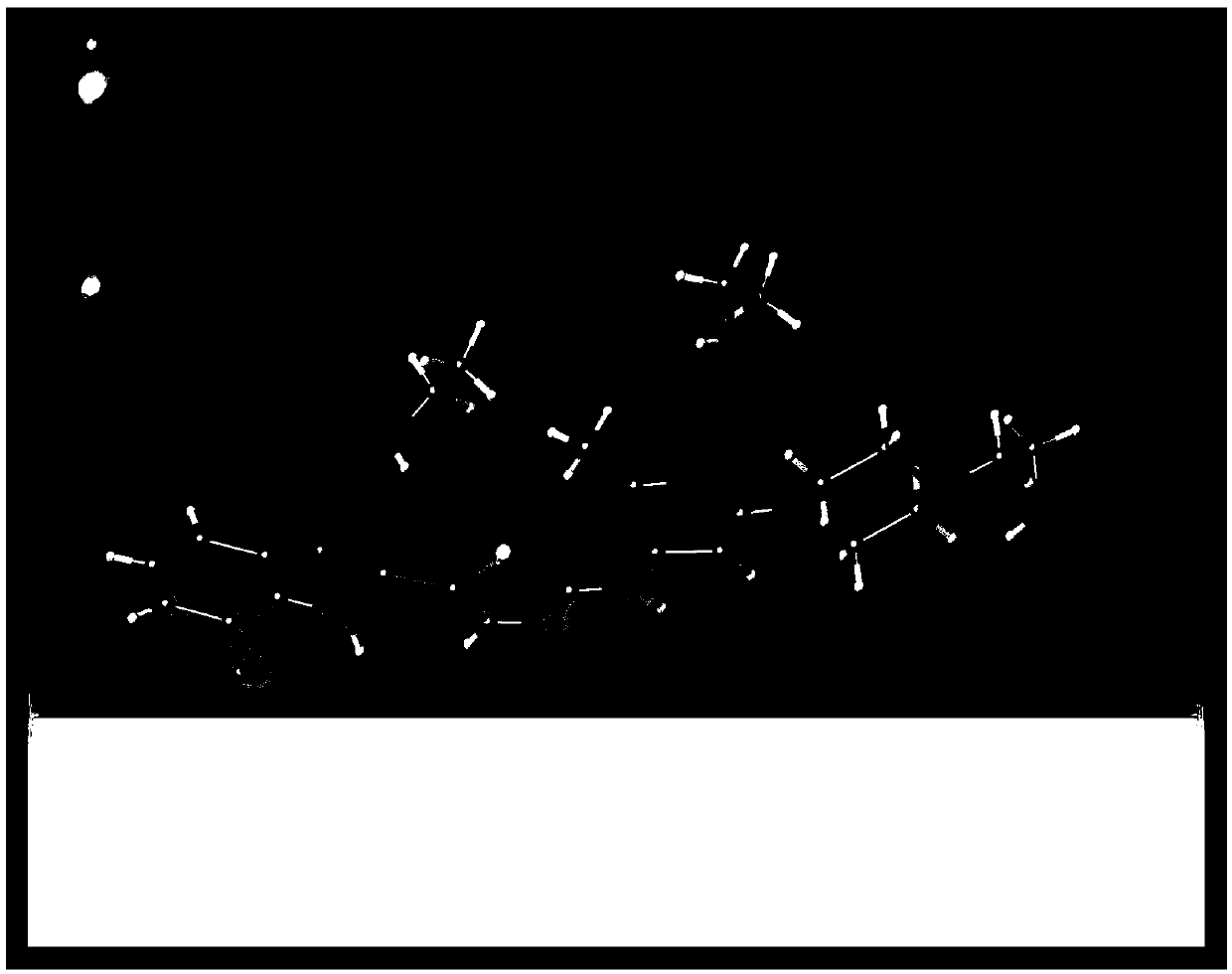
Image
Examples
Embodiment 1
[0043] (1) Add the crude product monohydrate of dasatinib into the absolute ethanol solution, wherein the concentration of dasatinib in the absolute ethanol solution is 0.1mg / mL;
[0044] (2) Ultrasonic heating was carried out in a desktop ultrasonic instrument to dissolve it, and the temperature was 35°C for 20 minutes;
[0045] (3) Slowly cool down to 0°C, filter, place in a small beaker, seal with parafilm, prick holes, volatilize, and crystallize for 20 minutes;
[0046] (4) Filtrate and dry under reduced pressure at 50°C to obtain a single crystal of dasatinib.
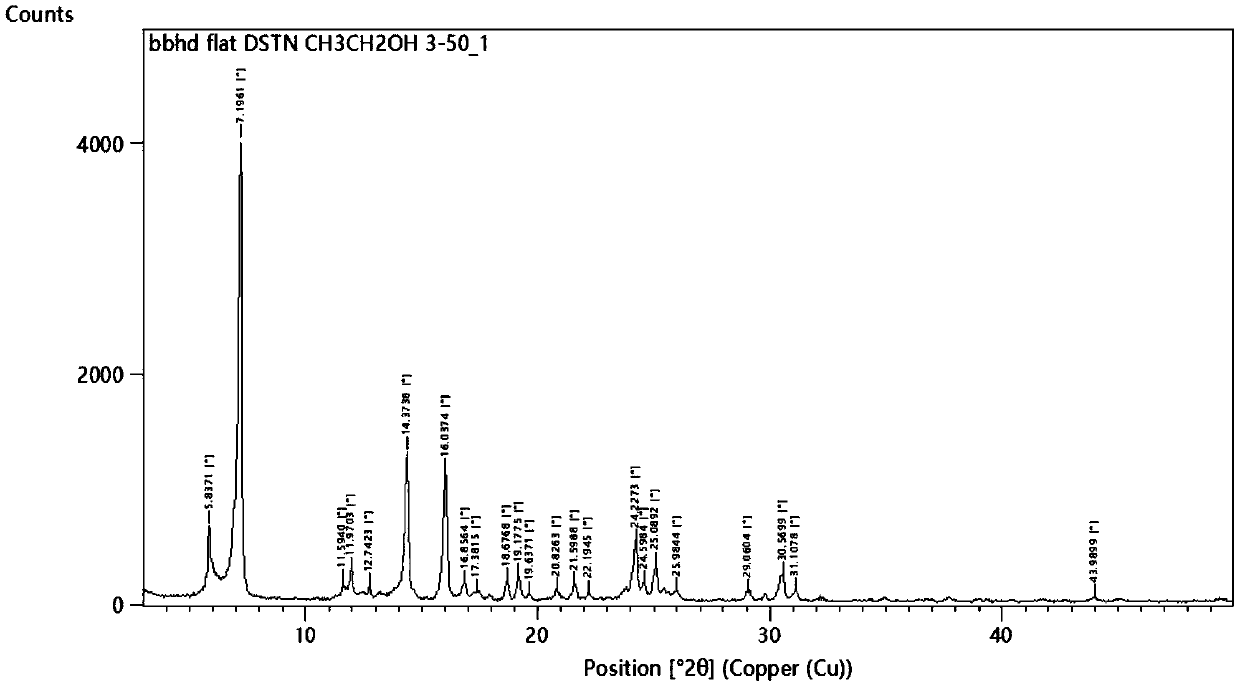
[0047] The product yield is 85.7%, and the product purity is 99.91%. After determination, its X-ray powder diffraction pattern and figure 1 Basically the same.
Embodiment 2
[0049] (1) Dasatinib monohydrate is added to the absolute ethanol solution, wherein the concentration of dasatinib in the absolute ethanol solution is 7 mg / mL;
[0050] (2) Ultrasonic heating is carried out in a desktop ultrasonic instrument to dissolve it, and the temperature is 45° C. for 1 hour;
[0051] (3) Slowly cool down to 35°C, filter, place in a small beaker, seal with parafilm, pierce holes, volatilize at room temperature, and crystallize for 10 hours;
[0052] (4) Filtrate and dry under reduced pressure at 40°C to obtain a single crystal of dasatinib.
[0053] The product yield is 90.5%, and the product purity is 99.98%. After determination, its X-ray powder diffraction pattern and figure 1 Basically the same.
Embodiment 3
[0055] (1) Dasatinib monohydrate was added to the absolute ethanol solution, wherein the concentration of dasatinib in the absolute ethanol solution was 9 mg / mL;
[0056] (2) Ultrasonic heating is carried out in a desktop ultrasonic instrument to dissolve it, and the temperature is 70° C. for 2 hours;
[0057] (3) Slowly cool down to 55°C, filter, place in a small beaker, seal with parafilm, prick holes, volatilize at room temperature, and crystallize for 48 hours;
[0058] (4) Filtrate and dry under reduced pressure at 60°C to obtain a single crystal of dasatinib.
[0059] The product yield is 88.8%, and the product purity is 99.95%. After determination, its X-ray powder diffraction pattern and figure 1 Basically the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com