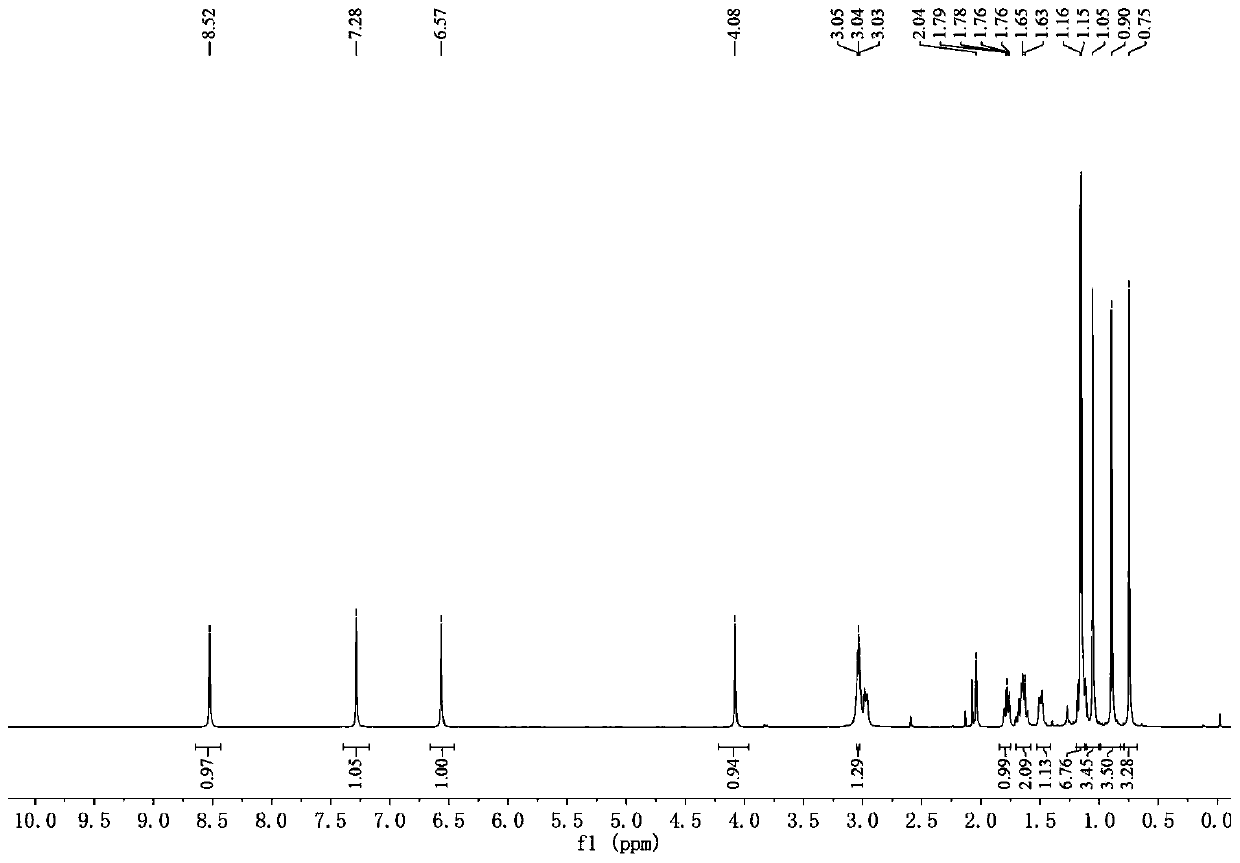
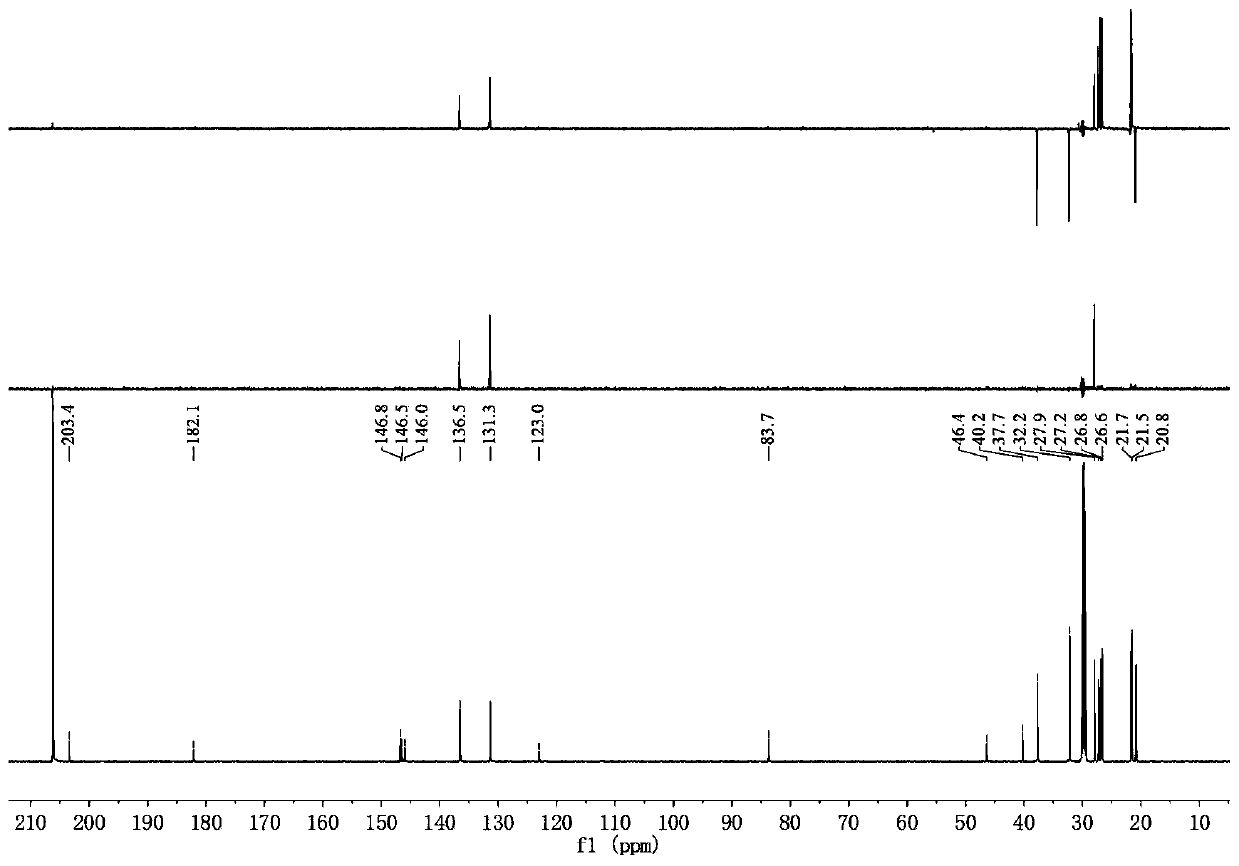
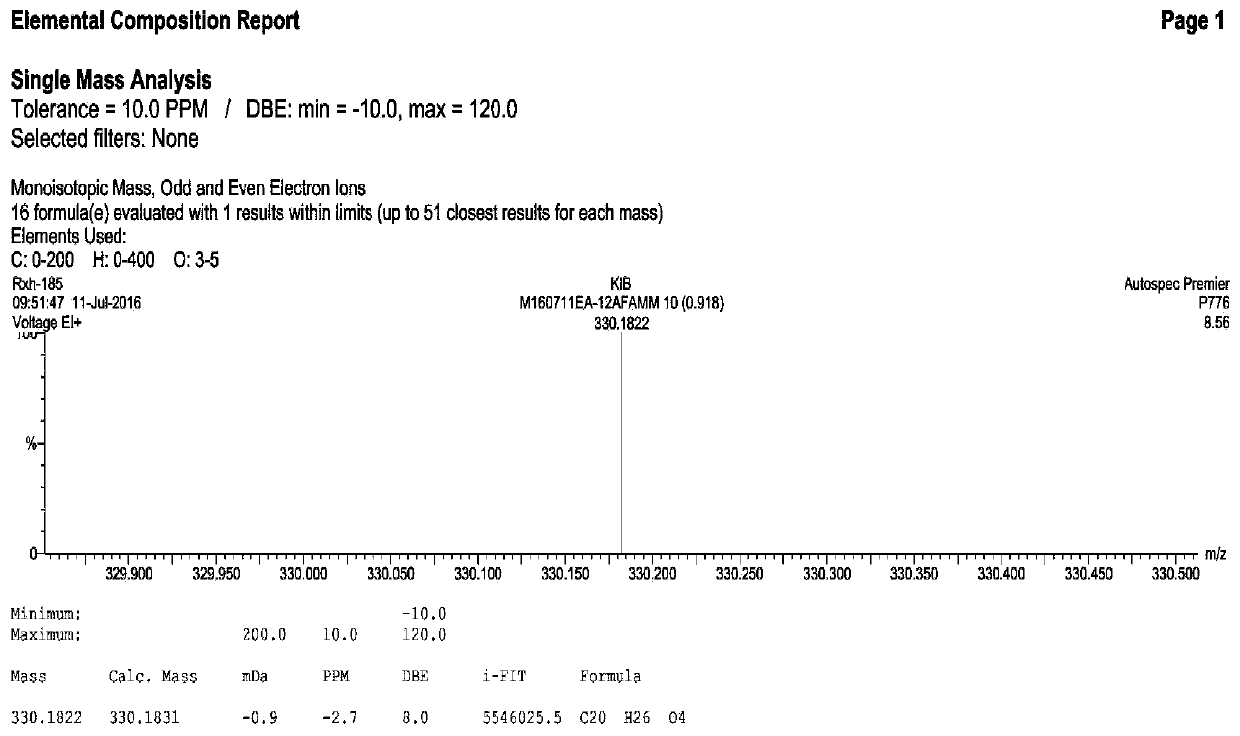
Abietane diterpenoid compound, preparation method thereof, pharmaceutical composition with antiplatelet activity and application thereof
A compound and diterpenoid technology, applied in the field of pharmaceutical compositions with antiplatelet activity, rosinane-type diterpenoids and their preparation fields, can solve the problem of platelet hyperreactivity, pharmacokinetics and pharmacodynamics Large differences, individual differences in drug efficacy, etc., to achieve significant in vivo antithrombotic activity, potent in vivo antithrombotic activity, and potent antiplatelet activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The present invention provides the preparation method of abietane-type diterpenoids described in the above technical scheme, comprising the following steps:
[0057] Sage Kangding is extracted with acetone, and the obtained extract is distilled under reduced pressure to obtain an extract;
[0058] The extract is subjected to silica gel column chromatography, and the eluent used is chloroform, and then the obtained chloroform part is subjected to silica gel column chromatography under gradient elution conditions, and the eluent used is a mixed solution of petroleum ether / chloroform / ethyl acetate , collected in turn to obtain 7 fractions, recorded as A~G fractions;
[0059] Mix the sample of component B with polyamide, pass it through the MCI column under gradient elution conditions, the mobile phase used is methanol / water mixture, and collect 8 components sequentially, which are recorded as components B1 to B8;
[0060] Under gradient elution conditions, the B5 componen...
Embodiment 1
[0080] The preparation method of compound 1~7, comprises the following steps:
[0081] Take the dried whole herb of Kangding Sage, pulverize it, extract it by cold soaking with acetone at room temperature for 3 times (48h / time), combine the obtained extracts, and distill under reduced pressure to obtain the extract;
[0082] Extraction extract was subjected to silica gel column chromatography, and eluted with chloroform; Gained chloroform fraction (667g) was carried out silica gel column chromatography, and gradient elution with petroleum ether / chloroform / ethyl acetate mixed solution (petroleum ether, chloroform and ethyl acetate The volume ratio is 50:1:1, 20:1:1, 10:1:1, 5:1:1, 1:1:1), and 7 components are collected by thin-layer chromatography. Recorded as A ~ G components;
[0083] Among them, component B (223g) was mixed with polyamide, passed through MCI column, and used methanol / water mixture as mobile phase gradient elution (the volume ratio of methanol and water was ...
Embodiment 2
[0119] The compound described in embodiment 1 is to the effect of rabbit platelet aggregation induced by arachidonic acid (AA):
[0120] 1. Solution preparation
[0121] AA solution: add the marked volume of pure water according to the instructions, fully dissolve the prepared AA solution with a concentration of 15mM, subpackage and refrigerate (-20°C) for later use;
[0122] Test sample (compound described in Example 1) solution or reference substance (aspirin) solution: use dimethyl sulfoxide (DMSO) preparation concentration to be the test sample mother solution or reference substance mother solution of 10mg / mL, then use DMSO was used to dilute and prepare the test sample solution or reference solution of the required concentration;
[0123] Ca-free 2+ Tyrode's solution (Ca 2+ free Tyrode's buffer): containing NaCl 137mM, KCl 2.68mM, MgSO 4 ·7H 2 O 0.2mM, NaH 2 PO 4 2H 2 O 0.42mM, NaHCO 3 11.9mM, glucose 5.05mM, EGTA 0.2mM, pH 6.5;
[0124] Tyrode's buffer (0.25%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com