TIM-3 single-domain antibody and application thereof
1. TIM-3, single-domain antibody technology, applied in the fields of antibodies, applications, antibody medical components, etc., can solve the problems of weakened affinity of single-domain antibodies, and achieve the effect of enhancing anti-tumor activity and improving tumor tissue permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0066] The specific steps of the present invention are illustrated below through the examples, but are not limited to the scope of the examples. The terms used in the present invention, unless otherwise specified, generally have the meanings commonly understood by those skilled in the art. The present invention will be further described in detail below in conjunction with specific examples and with reference to data. It should be understood that these examples are only for illustrating the present invention, rather than limiting the scope of the present invention in any way. In the following examples, various procedures and methods not described in detail are conventional methods well known in the art.
[0067] The present invention will be further described below in conjunction with specific examples, but the protection scope of the present invention is not limited thereto.
Embodiment 1
[0068] Example 1 Construction and screening of phage-displayed TIM-3 single domain antibody library
[0069] New Zealand white rabbits were immunized with TIM-3 recombinant protein (purchased from Beijing Yiqiao Shenzhou Technology Co., Ltd.), the spleen was taken, total RNA was extracted, and the antibody variable region gene was amplified by reverse transcription, and the antibody gene was amplified The fragments were ligated with phage vectors, purified, and the ligated products were electrotransformed into TG1 competent cells to prepare a library of TIM-3 single domain antibodies with a capacity of 6×10 9 . At the same time, the synthetic human VH library (refer to Thomas Tiller, et al. A fully synthetic human Fabantibody library based on fixed VH / VL framework pairings with favorable biophysical properties. mAbs 5:3, 445–470; May / June 2013) was linked together with the phage vector, After purification, the ligated product was electrotransformed into TG1 competent cells. ...
Embodiment 2
[0070] Example 2 Prokaryotic expression and purification of single domain antibody
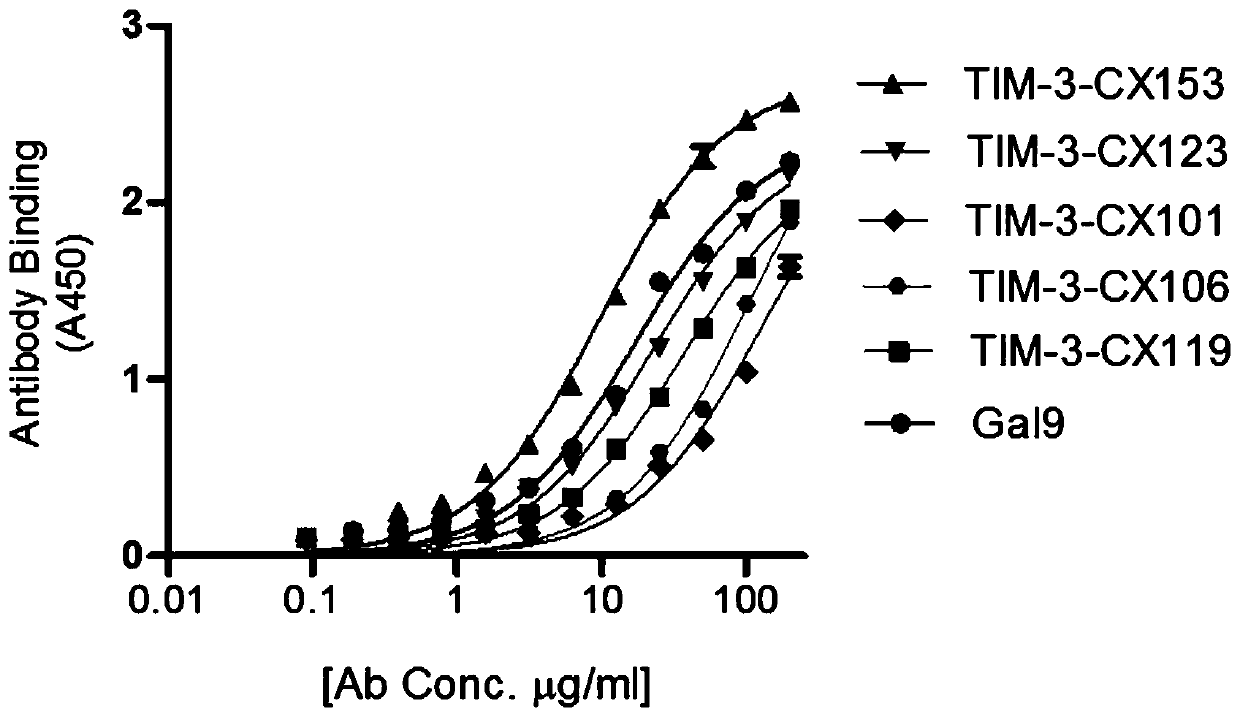
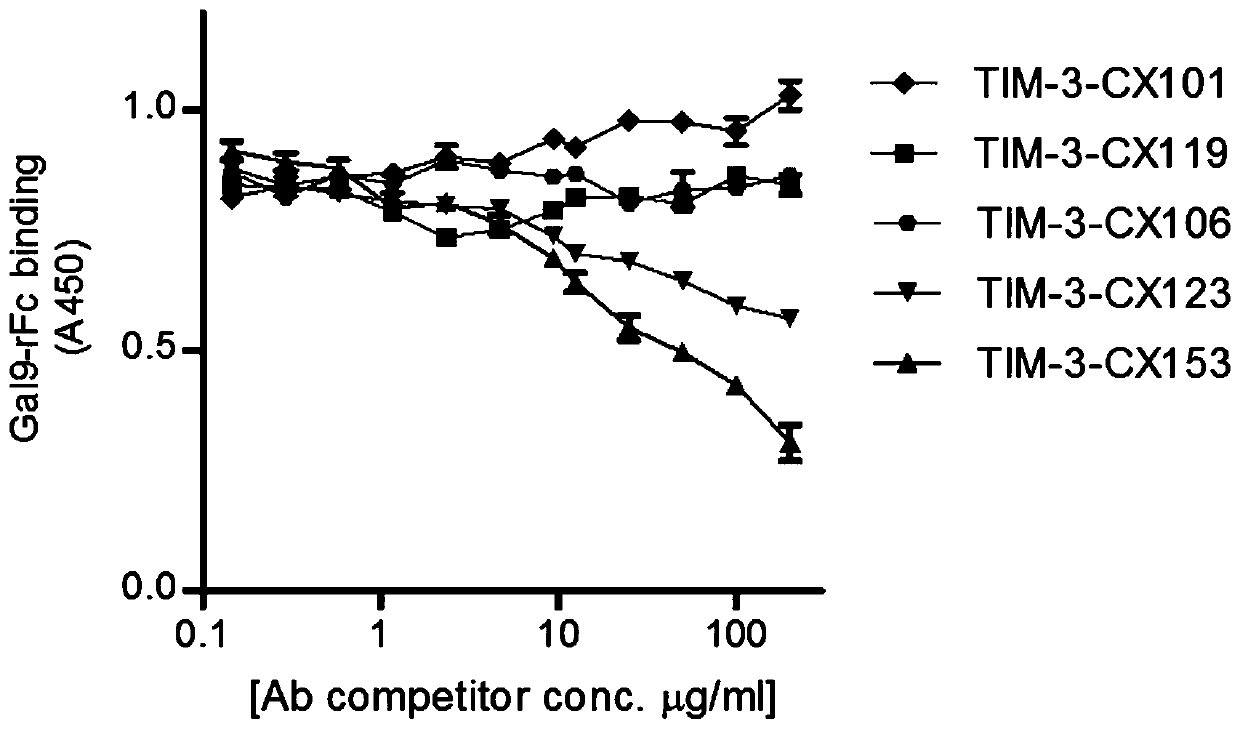
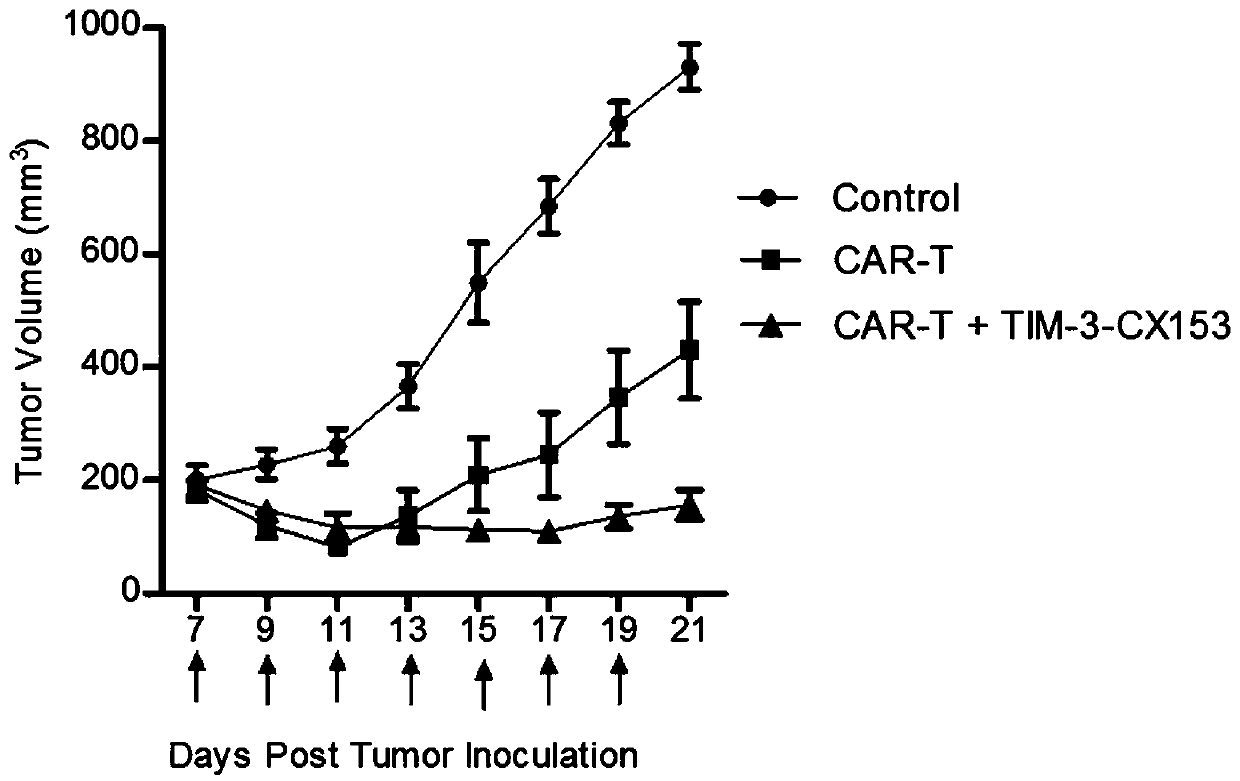
[0071] The single domain antibody gene obtained in Example 1 was subcloned into the prokaryotic expression vector pET28, and introduced into the expression strain BL21, and a single colony was picked from the overnight-grown ampicillin plate, induced by adding IPTG to the LB medium, and induced for 8 hours at 30°C . After the wall was broken by ultrasound, the supernatant was collected by centrifugation, and purified by His nickel column to obtain single domain antibodies TIM-3-CX101 (SEQ ID NO:1), TIM-3-CX106 (SEQ ID NO:2), TIM-3 - CX119 (SEQ ID NO:3), TIM-3-CX123 (SEQ ID NO:4), TIM-3-CX153, (SEQ ID NO:5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com