Muscone 3-position derivative, and preparation method and application thereof
A technology of musk ketone and derivatives, applied in the field of medicine, can solve few problems and achieve the effect of broad medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of formula I and formula II compound
[0063] 1.1: Preparation of compound 2
[0064]
[0065] Dissolve compound 1 (cyclopentadecanone, 6.72g, 30mmol), p-toluenesulfonic acid (570mg, 3mmol) in toluene (90mL) and ethylene glycol (83mL, 1500mmol), then reflux for 2 hours to end the reaction After the reaction solution was cooled to room temperature, it was transferred to a separatory funnel for liquid separation, and the separated toluene layer was successively washed with saturated NaHCO 3 , water, and saturated NaCl, dried over anhydrous magnesium sulfate, filtered with suction, and distilled off the solvent under reduced pressure to obtain a colorless oily substance, namely compound 2, with a yield of 99%.
[0066] Tested: 1 H NMR (400MHz, CDCl 3 ): δ3.91(s,4H), 1.66–1.53(m,4H), 1.46–1.23(m,24H).
[0067] 1.2: Preparation of Compound 3
[0068]
[0069] Compound 2 (8.05g, 30mmol) and liquid bromine (4.8g, 30mmol) were dissolved ...
Embodiment 2
[0129] Example 2: In Vitro Pharmacological Activity Screening of Muscone 3-Derivatives
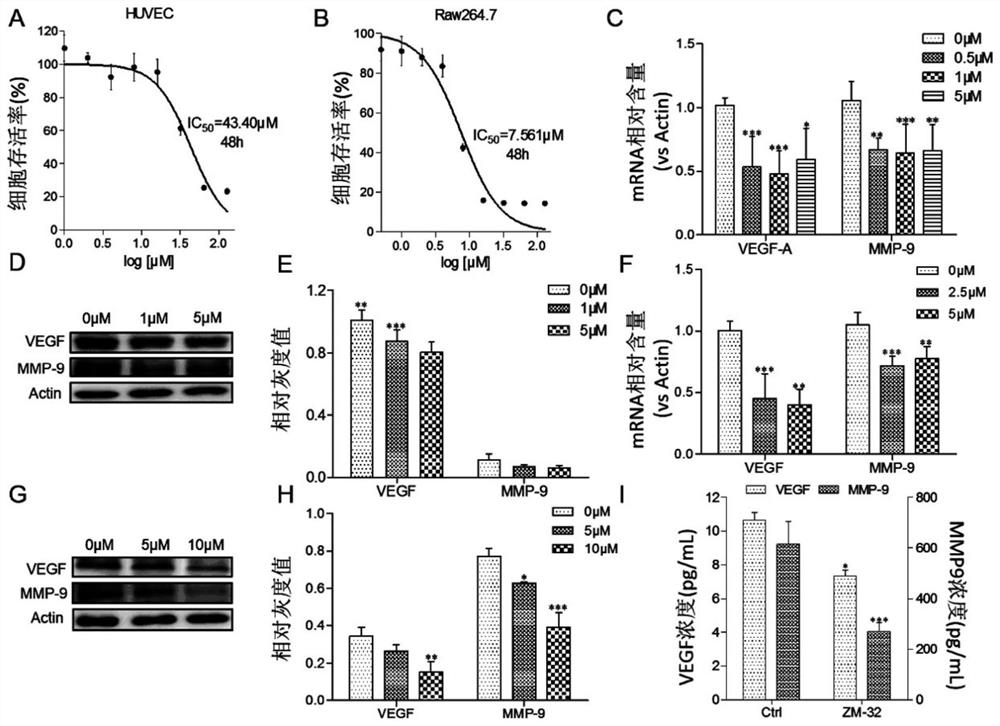
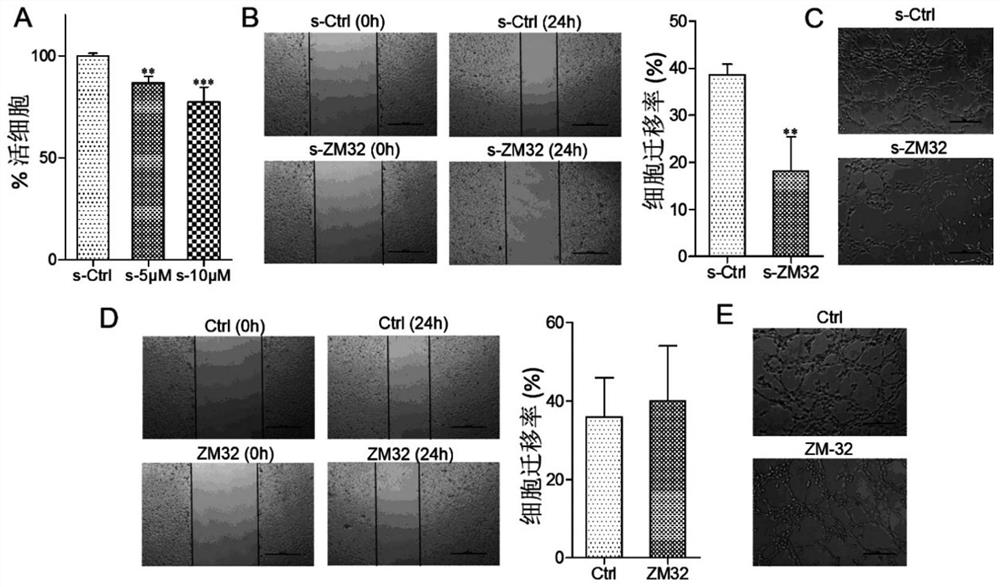
[0130] Macrophages play a potentially key regulatory role in complex angiogenesis and remodeling processes, and literature research results show that macrophages are the main source of angiogenesis-related factor VEGF-A, and MMP9 produced by macrophages can also Promotes the release of VEGF from its cellular stores and regulates angiogenesis. Therefore, the present invention conducts preliminary drug screening by measuring the effect of the target compound on the expression of VEGF-A mRNA in macrophages. Table 1 shows the activity results of the muscone 3-position derivatives of the present invention acting on Raw264.7 cells for 3 hours at a concentration of 1 μM on VEGF-A mRNA.
[0131] Table 4 Preliminary Screening of the Effects of Muscone 3-Position Derivatives on Raw264.7 Cells on VEGF-A mRNA
[0132]
[0133]
[0134] It can be seen from Table 4 that the 3-position derivative...
Embodiment 3
[0135] Example 3: Toxicity screening and analysis of muskone 3-position derivatives on cancer cells
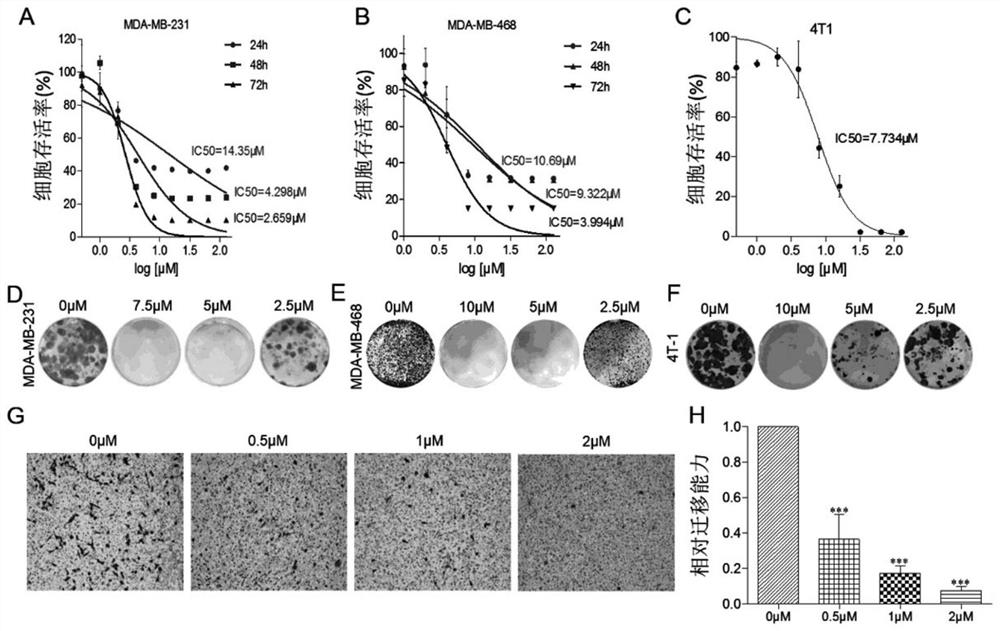
[0136] As can be seen from Table 4 in Example 2, compound: these 8 compounds of ZM-31, ZM-32, ZM-39, ZM-40, ZM-41, ZM-43, ZM-44, ZM-46 The inhibitory effect of VEGF-A mRNA expression is the most obvious. In this example, these 8 compounds were screened for MTT cytotoxicity of cancer cells. In this experiment, 6 kinds of solid tumor adherent cell lines were selected, which were human gastric cancer cell AGS, human breast Cancer cells MDA-MB-231, MDA-MB-468, human lung cancer cells A549, H1299 and human prostate cancer cell line PC-3, the results are shown in Table 5.
[0137] Table 5 The 3-position derivative of muscone acts on the IC50 value of cancer cells for 48 hours
[0138] compound AGS MDA-MB-231 MDA-MB-468 A549 H1299 PC-3 ZM-31 111.70±1.34 104.10±1.06 >128 23.33±1.34 64.81±1.21 105.10±1.07 ZM-32 8.36±1.04 4.29±1.18 9.32±1.13 8.15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com