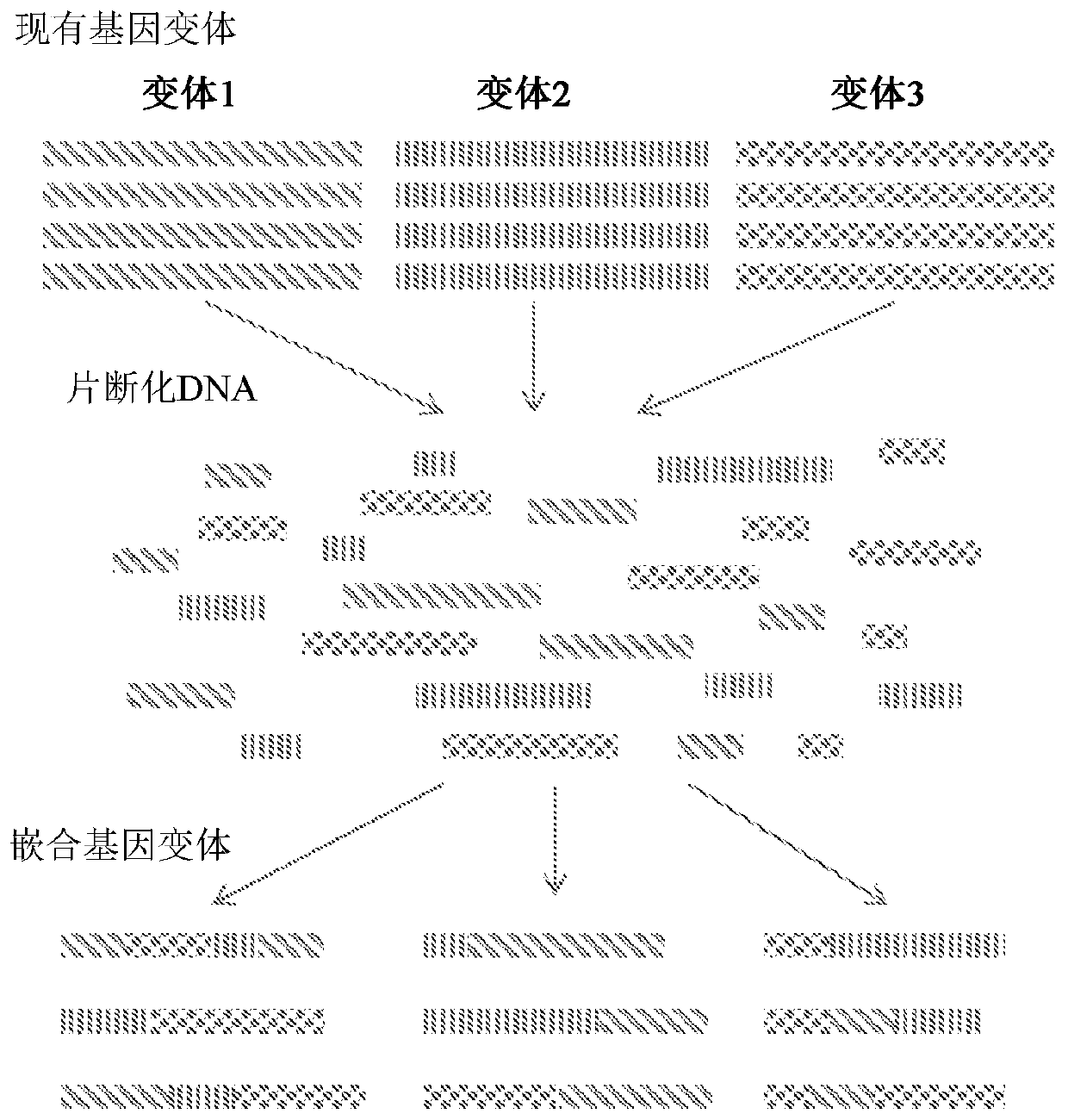
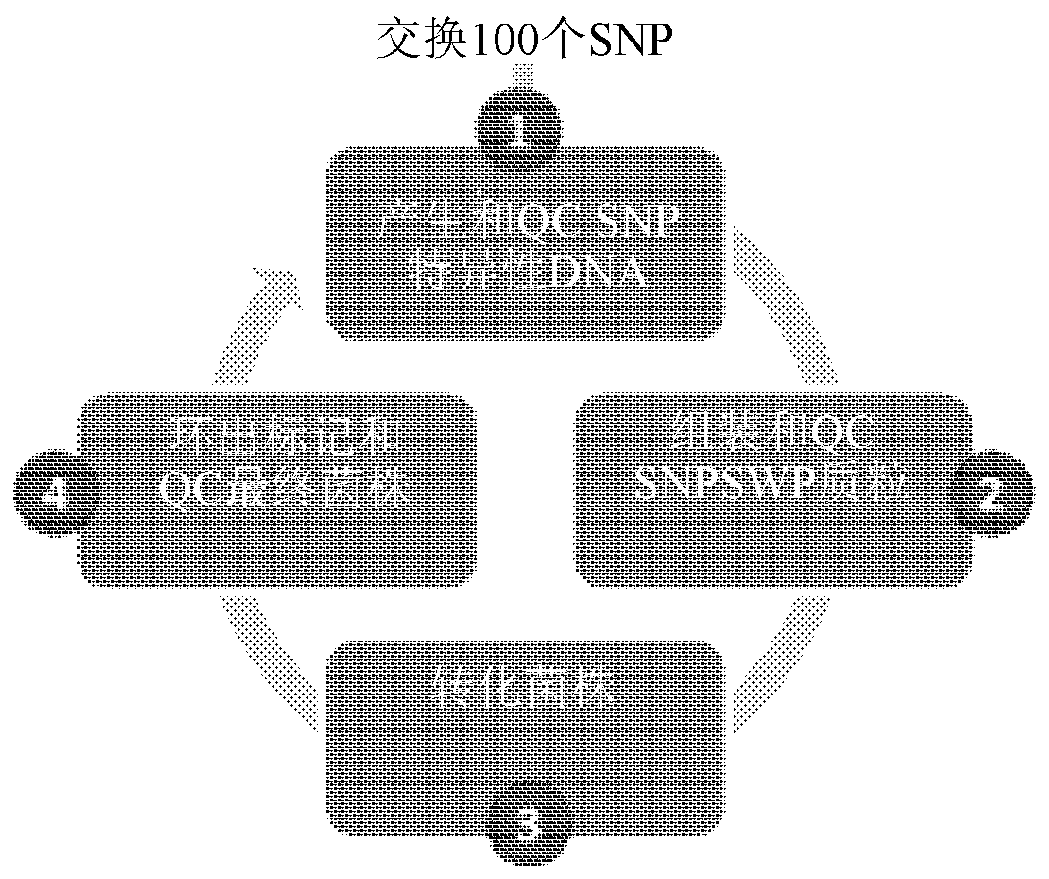
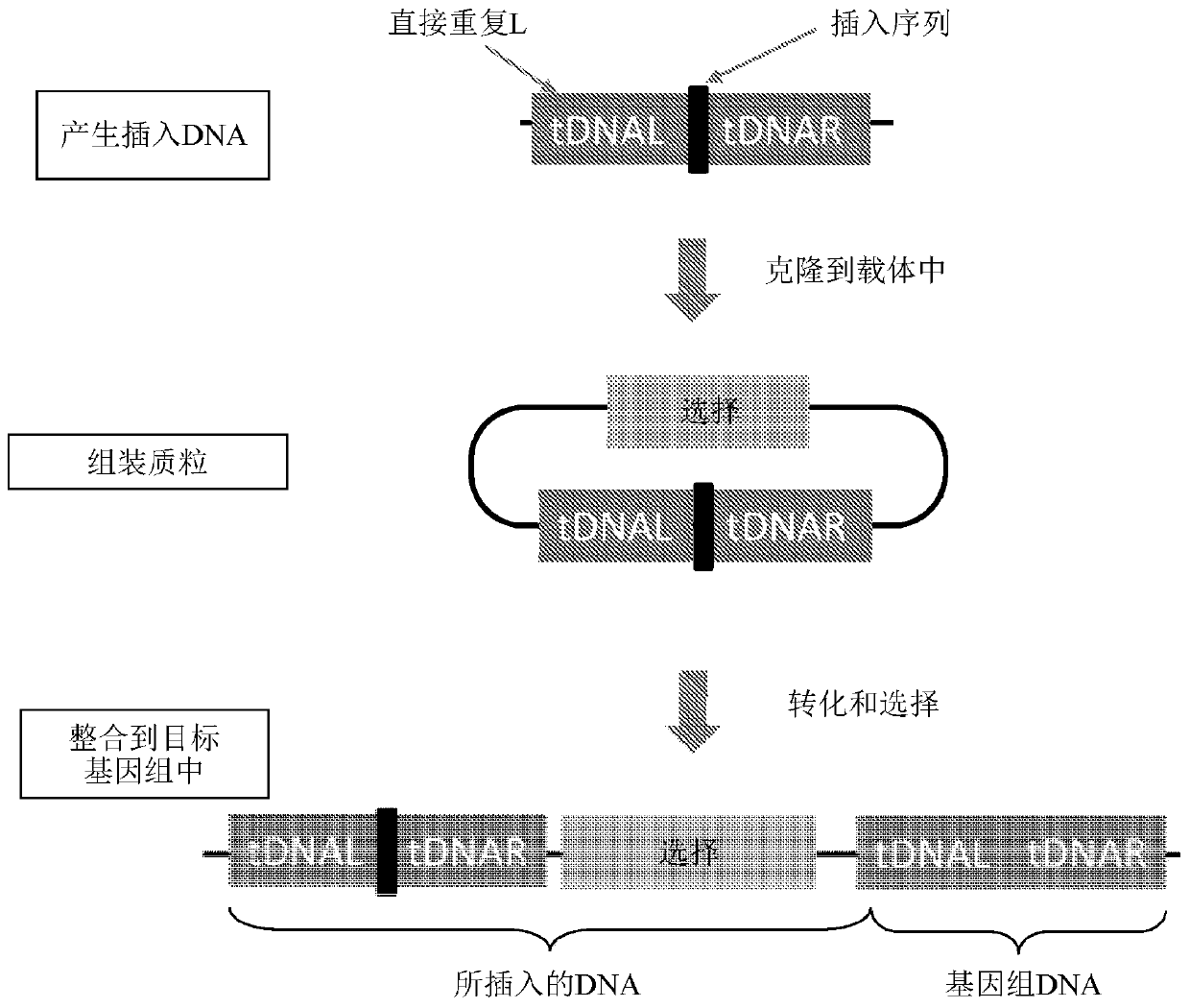
HTP genomic engineering platform for improving escherichia coli
A technology of Escherichia coli and genetic engineering, which is applied in the field of high-throughput microbial genetic engineering and can solve the problem that researchers do not have genetic engineering tools.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0898] The following examples are provided to illustrate various embodiments of the present disclosure and are not intended to limit the present disclosure in any way. Those skilled in the art will recognize that changes therein and other uses are encompassed within the spirit of the disclosure as defined by the scope of the claims.
[0899] Specifically, Examples 1-9 are HTP genetic engineering platforms in coryneform bacteria. However, a similar procedure has been tailored for E. coli and was successfully performed by the present inventors.
[0900] A brief table of contents is provided below to assist the reader only. This list is not intended to limit the scope of the examples or disclosure of this application.
[0901] Table 5.1 - Table of Contents for Example Sections
[0902]
[0903]
example 1
[0904] Example 1: Demonstration of HTP transformation and SNP library creation of coryneform bacteria
[0905] This example illustrates an embodiment of the HTP genetic engineering method of the present disclosure. Host cells are transformed with multiple SNP sequences of different sizes, all targeting different regions of the genome. The results demonstrate that the methods of the present disclosure are capable of producing rapid genetic changes of any kind throughout the entire genome of a host cell.
[0906] A. Cloning of Transformation Vectors
[0907] A variety of SNPs were randomly selected from Corynebacterium glutamicum (ATCC21300) and cloned into a Corynebacterium cloning vector using yeast homologous recombination cloning technology to assemble the vector, wherein each SNP is flanked by a direct repeat region, as described above in "Assembly / Cloning of Custom Plasmids" section and as described in image 3 described in.
[0908] The SNP cassette used in this exam...
example 2
[0929] Example 2: HTP Genome Engineering - Implementation of SNP Libraries to Repair / Improve Industrial Microbial Strains
[0930] This example illustrates several aspects of the SNP exchange library in the HTP strain improvement program of the present disclosure. In particular, the examples illustrate several conceived approaches to rehabilitate currently existing industrial strains. This example describes up-swing and down-swing approaches to explore the phenotypic solution space arising from multiple genetic differences that may exist between "basic", "intermediate" and industrial strains.
[0931] A. Identification of SNPs in Diversity Pools
[0932] An exemplary strain improvement program using the methods of the present disclosure was performed on an industrial microbial strain (referred to herein as "C"). The diversity pool strains used in this procedure are indicated by A, B and C. Strain A represents the original production host strain prior to any mutagenesis. St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com