Polypeptide, uses thereof in preparation of drugs, and drugs
A technology of drugs and peptides, applied in the field of medicine, can solve problems that need to be studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
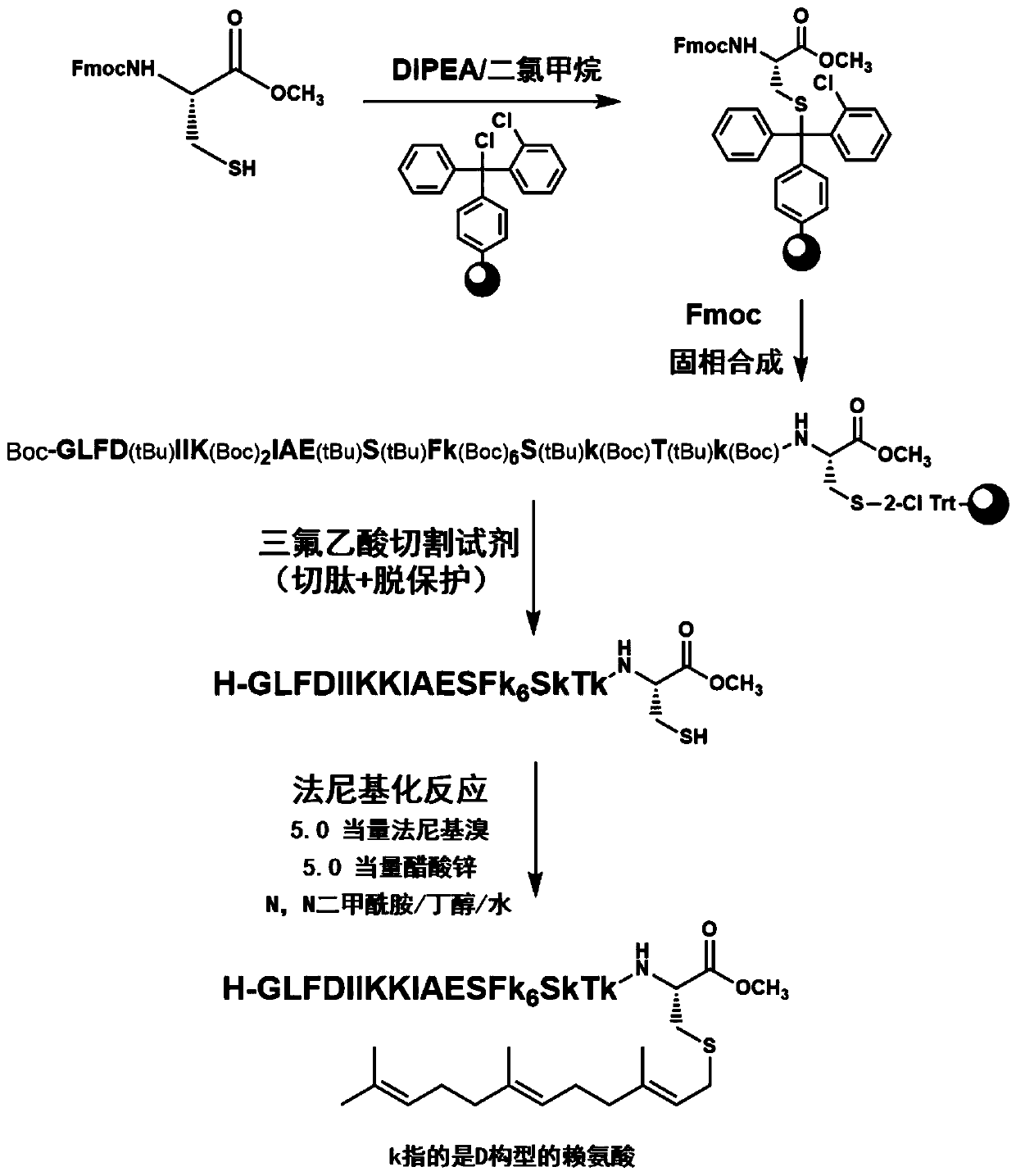
[0061] The preparation of embodiment 1 compound
[0062] The first amino acid Fmoc-Cys-OMe for solid-phase synthesis can be obtained by purifying the commercially purchased amino acid Fmoc-Cys(Trt)-OH through a two-step reaction, and combined with 2-Cl Chlorotrityl resin for solid-phase synthesis Linked under the action of organic base DIPEA (see literature Diaz-Rodriguez V., et al., Synthesis of Peptides Containing C-Terminal Methyl Esters Using Trityl Side-Chain Anchoring: Application to the Synthesis of a-Factor and a-Factor Analogs. OrganicLetters,2012,14(22),5648-5651.), and obtain the desired polypeptide sequence by Fmoc solid-phase synthesis method, next, use TFA / thioanisole / H 2 The mixed reagent of O / phenol / 1,2-ethanedithiol (82.5 / 5 / 5 / 5 / 2.5, v / v) cuts the polypeptide from the resin and removes the protecting groups of all amino acids, and precipitates in ether After obtaining the crude peptide, dissolve the obtained crude peptide in DMF / BuOH / H 2 O (2 / 1 / 1, v / v), after...
Embodiment 2
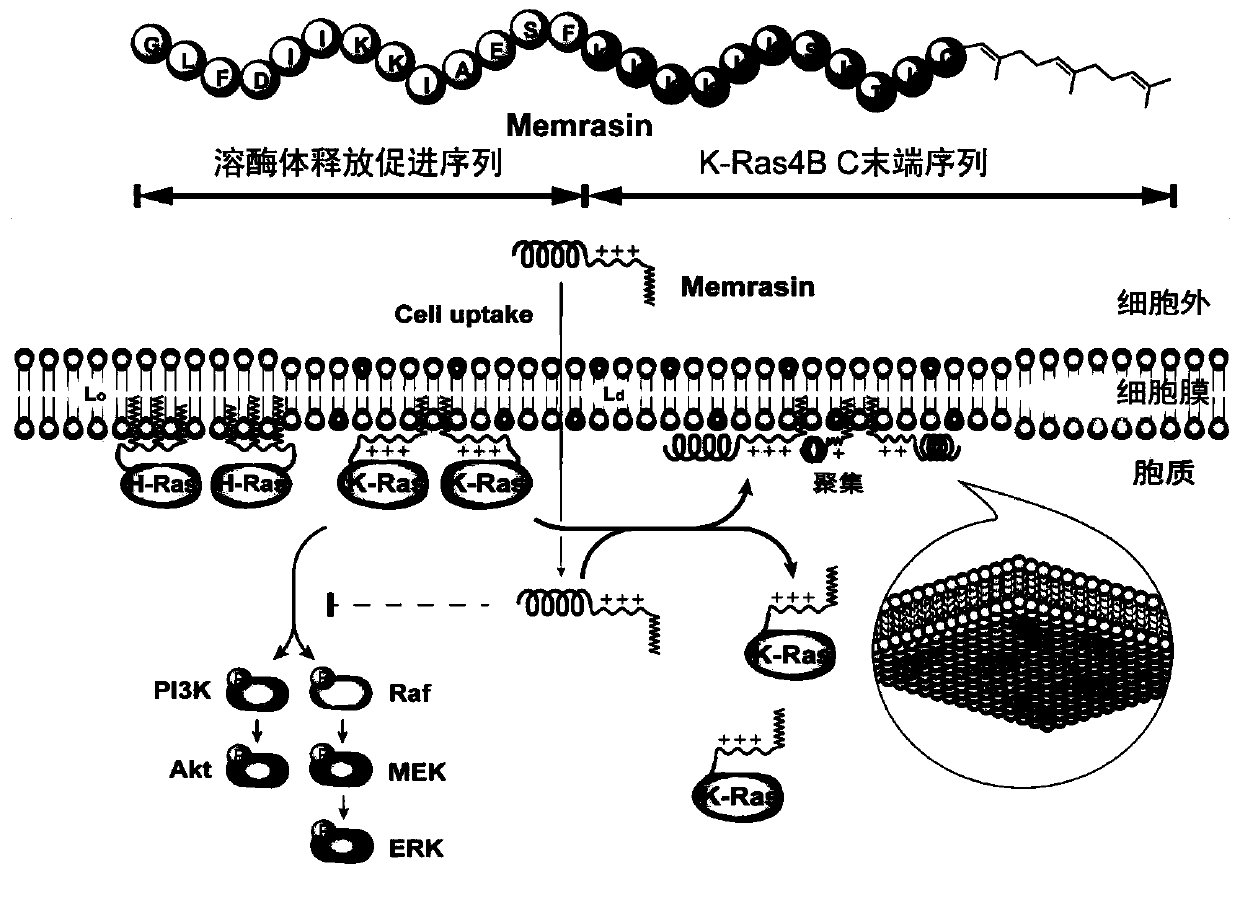
[0067] Example 2 Experiment of farnesylated anticancer polypeptide regulating K-Ras4B localization
[0068] 1. Construction and related characterization of cell lines stably expressing mCitrine fluorescent protein-tagged K-Ras4B protein
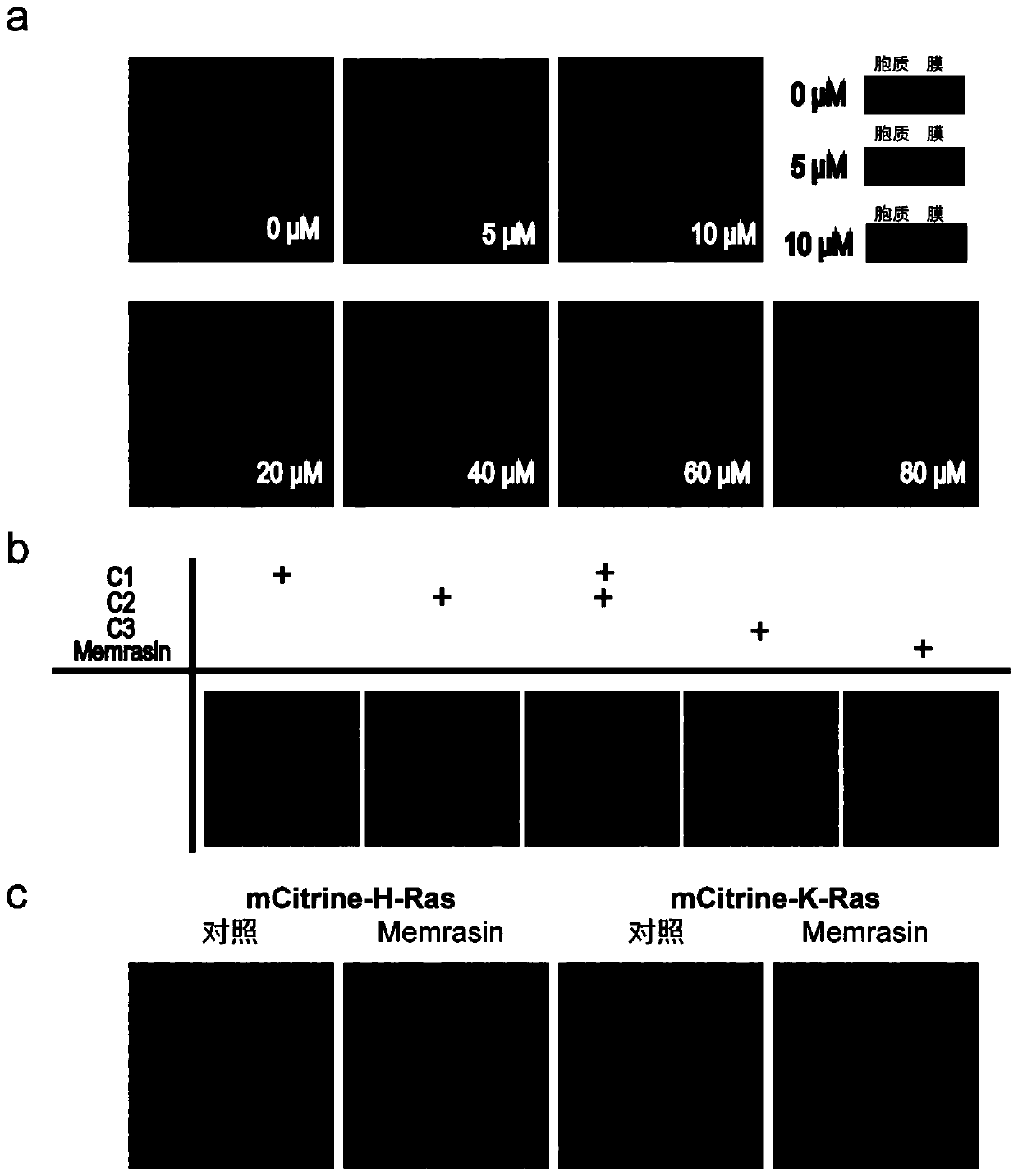
[0069]The plasmid carrying the mCitrine-K-Ras4B gene was transfected into the MDCK cell line with a transfection reagent (lipofectamine 2000, purchased from Thermo Company), and monoclonal cells were obtained by sorting by flow cytometry, and an appropriate cell line was selected for Expand the culture to obtain a cell line stably expressing fluorescent protein-tagged K-Ras4B protein. The above cell lines were inoculated into a 6-well plate with coverslips (seeding density 100%), and the prepared farnesylated anti-cancer polypeptide Memrasin was added to the serum-containing medium to prepare a final concentration of 0.5 , 10, 20, 40, 60, 80μM solution, then add the corresponding cells, incubate at 37°C for 10 minutes, suck out the working s...
Embodiment 3
[0074] Example 3 Mechanism experiment of farnesylated anti-cancer polypeptide changing K-Ras4B localization
[0075] 1. Intracellular localization of farnesylated anticancer peptides
[0076] A fluorescein-labeled FAM-Memrasin polypeptide was synthesized to observe the localization of farnesylated anti-cancer polypeptides in cells. The polypeptide sequence is H-GLFDIIKKIAESF-K(FAM)K 5 SMTKC(Far)-OMe. Inoculate A549 cells into a 6-well plate with coverslips and dissolve the FAM-Memrasin polypeptide in the cell culture medium to prepare a working solution with a final concentration of 1 μM and add it to the cells, incubate at 37°C for 10 minutes and wash with phosphate buffered saline After the cells were fixed and sealed, the localization of the fluorescent polypeptide in the cells was observed under a confocal microscope. The results were as follows: Figure 4 a. The results show that the fluorescently labeled Memrasin polypeptide has a clear localization on the cell membra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com