Nesiritide acetate peptide mapping analysis method and application thereof
An analysis method, nesiritide technology, applied in the field of drug analysis, can solve the problems of analysis deviation, time-consuming, and peaks appearing in front, and achieve the effect of short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
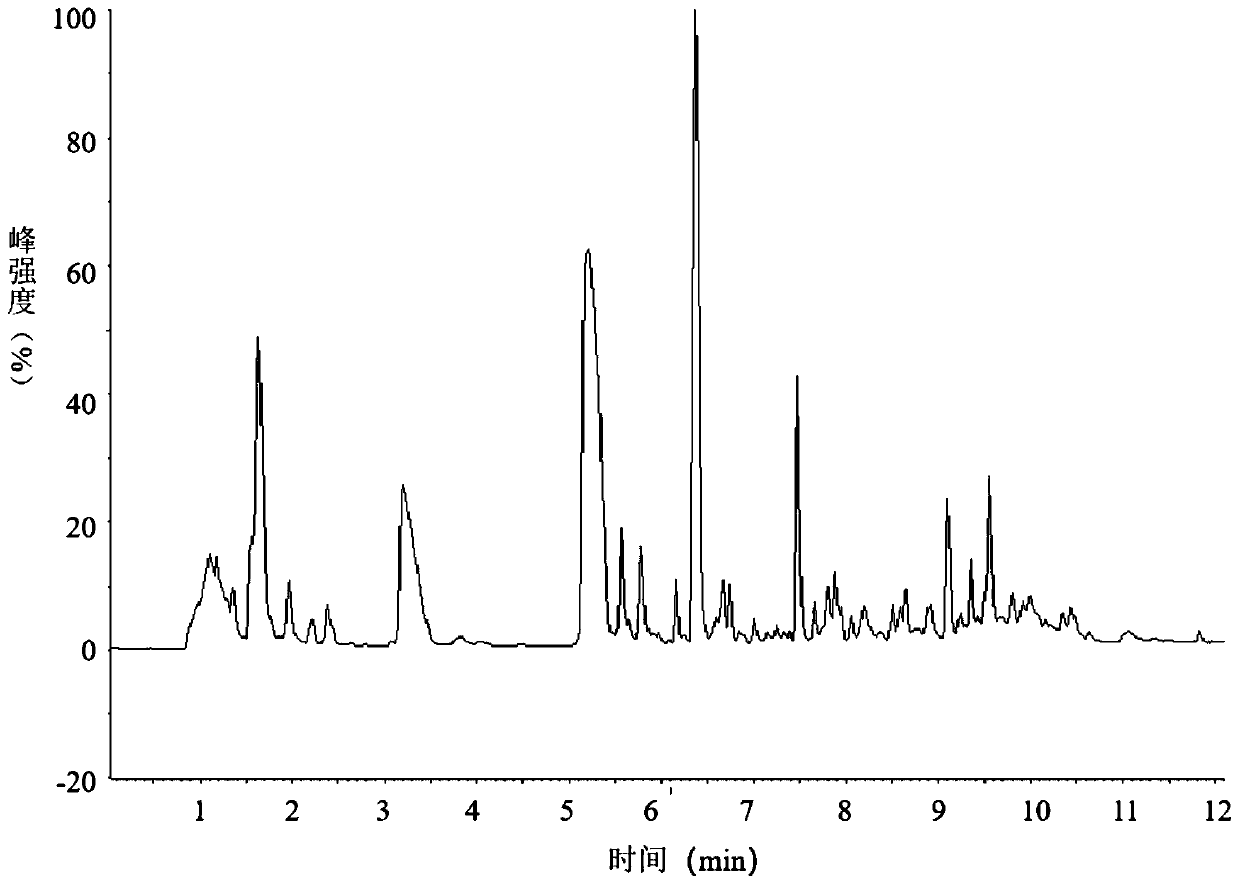
[0056] This embodiment provides a method for analyzing the peptide map of nesiritide, which specifically includes the following steps:
[0057] (1) Reduction and alkylation of nesiritide samples
[0058] Dilute the sample with water to 1.5mg / mL and add it to a 1.5mL centrifuge tube, add 100μL 0.1mol / L ammonium bicarbonate buffer, then add 11.56μL 0.1mol / L DTT (dithiothreitol), mix well, and Incubate at 56° C. for 1 h, and after incubation, place it in a water bath at 20° C. to cool for 1 min.
[0059] Add 12.6 μL of 0.25 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 20° C. in the dark for 1 h.
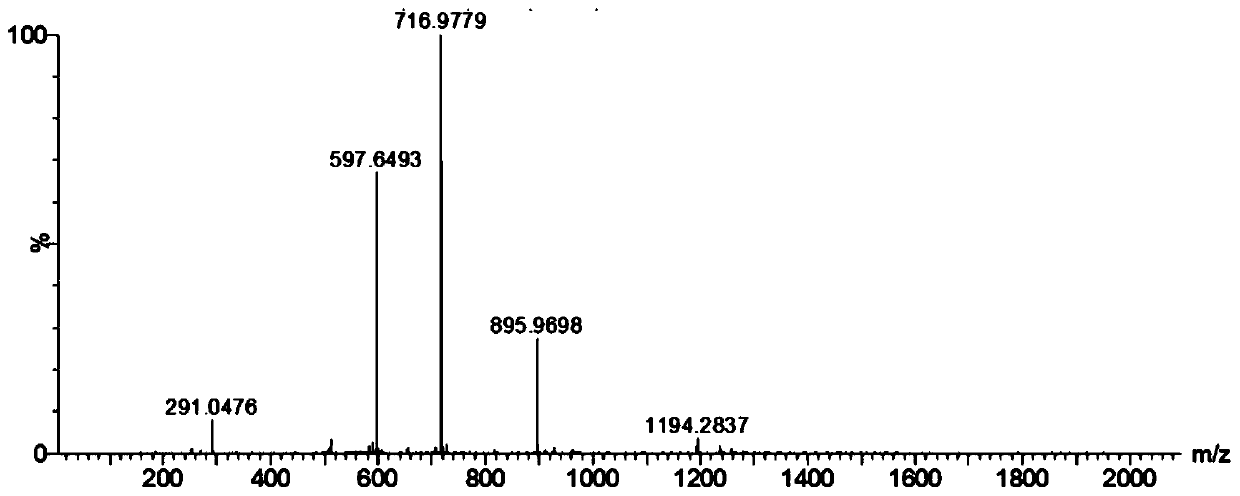
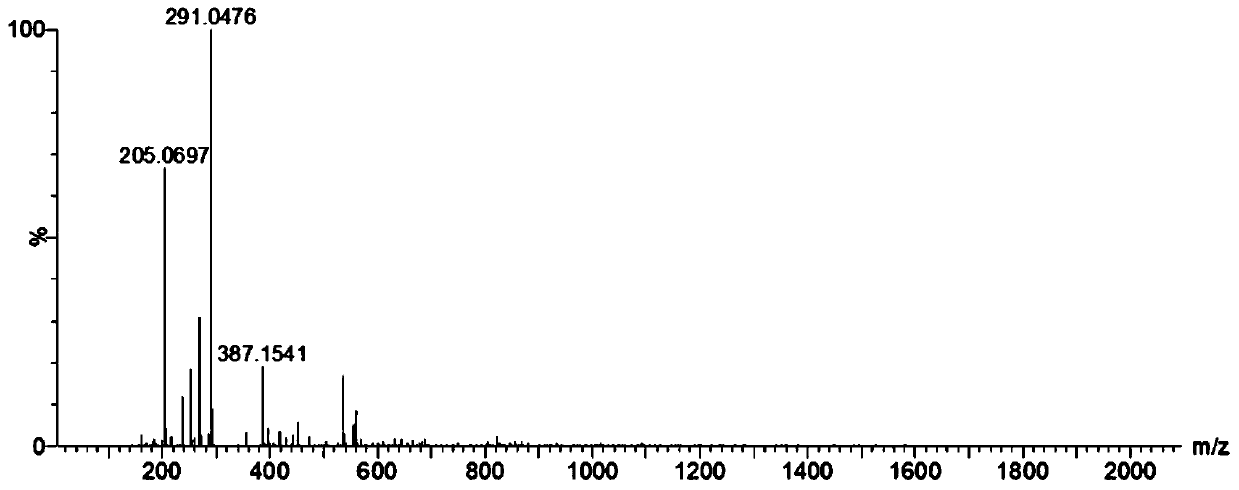
[0060] After the reaction, use mass spectrometry to confirm that the reduction and alkylation are complete (such as figure 1 shown), four mass spectrum peaks can be observed in the figure, and figure 2 The comparison of the mass spectrum of nesiritide without reduction and alkylation shows that the reduction and alkylation are complete, and the disul...
Embodiment 2
[0074] This embodiment provides a method for analyzing the peptide map of nesiritide, which specifically includes the following steps:
[0075] (1) Reduction and alkylation of nesiritide samples
[0076] Dilute the sample with water to 1.5mg / mL and add it to a 1.5mL centrifuge tube, add 100μL 0.4mol / L ammonium bicarbonate buffer, then add 11.56μL 0.4mol / L DTT (dithiothreitol), mix well, and Incubate at 56° C. for 1 h, and after incubation, place it in a water bath at 25° C. to cool for 1 min.
[0077] Add 12.6 μL of 0.5 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 25° C. in the dark for 1 h.
[0078] After the reaction, MS detection was used to confirm that the reduction and alkylation were complete (such as Figure 4 shown), four mass spectrum peaks can be observed in the figure, and figure 2 The comparison of the mass spectrum without reduction and alkylation in the above shows that the reduction and alkylation are complete, and the disulfi...
Embodiment 3
[0089] This embodiment provides a method for analyzing the peptide map of nesiritide, which specifically includes the following steps:
[0090] (1) Reduction and alkylation of nesiritide samples
[0091] Dilute the sample with water to 1.5mg / mL and add it to a 1.5mL centrifuge tube, add 100μL 0.2mol / L ammonium bicarbonate buffer, then add 11.56μL 0.2mol / L DTT, mix well, and incubate at 56°C for 1h, incubate Afterwards, it was placed in a 15°C water bath to cool for 1 min.
[0092] Add 12.6 μL of 0.5 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 15° C. in the dark for 1 h.
[0093] After the reaction, MS detection was used to confirm that the reduction and alkylation were complete (such as Figure 4 shown), four mass spectrum peaks can be observed in the figure, and figure 2 The comparison of the mass spectrum without reduction and alkylation in the above shows that the reduction and alkylation are complete, and the disulfide bond of nesiriti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com