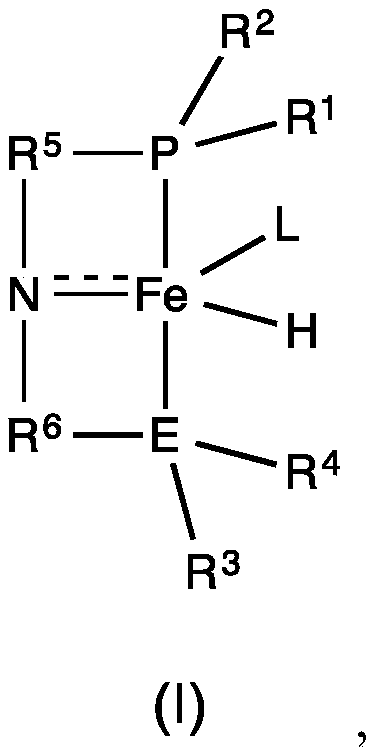
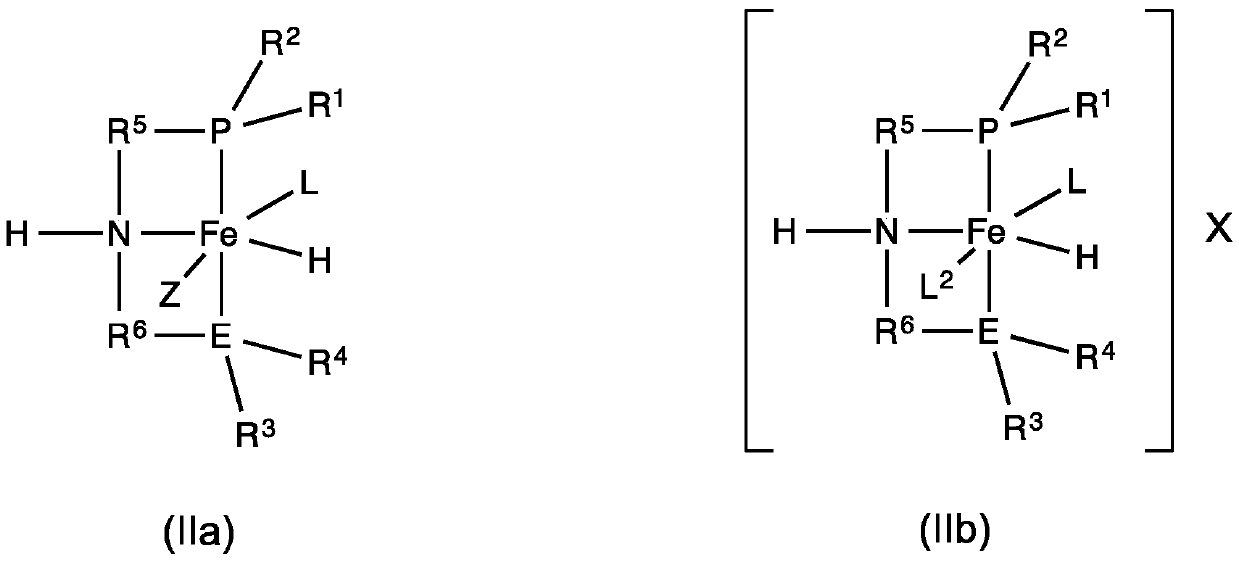
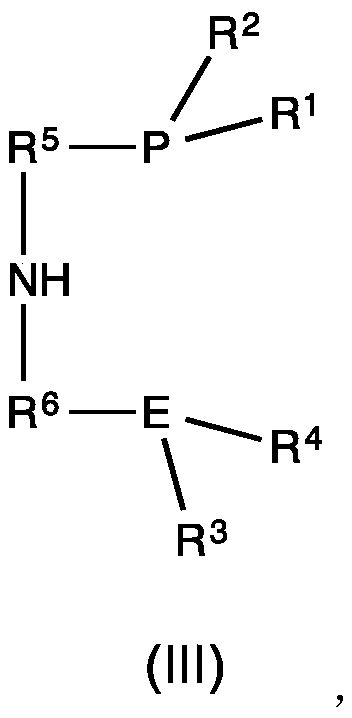
Iron-catalyzed transfer hydrogenation of esters to alcohols
一种催化剂、预催化剂的技术,应用在有机化学领域,能够解决昂贵、高氢气压力等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0114] General experiment information
[0115] Unless otherwise stated, all organometallic compounds were prepared and handled under a nitrogen atmosphere using standard Schlenk and glove box techniques. Anhydrous EtOH (200 proof alcohol) and toluene were purchased from Sigma Aldrich and used Molecular sieve preservation. Both EtOH and toluene were freshly distilled before use. Dimethyl 1,4-cyclohexanedicarboxylate (DMCD, mixture of cis and trans isomers, >90% purity) was purchased from Alfa Aesar and used without further purification. Compounds 1a-c have been previously reported in the literature. They were synthesized according to procedures slightly modified from literature procedures.
example 1
[0117] 1a[( iPr Synthesis of PNHP)Fe(H)(CO)(Br)]
[0118]
[0119] In a glove box, under a nitrogen atmosphere, a 200-mL oven-dried Schlenk flask was charged with the complex [ iPr PNHP]FeBr 2 (CO) (850mg, 1.545mmol), NaBH 4 (60 mg, 1.545 mmol, 98% purity) and 100 mL of dry EtOH. The resulting yellow solution was stirred at room temperature for 18 hours and filtered through Celite. The filtrate was evaporated to dryness to afford pure 1a (86% isolated yield). 1a 1 H and 31 P{ 1 H} NMR spectra are in good agreement with reported values (see S. Chakraborty et al., J. Am. Chem. Soc. 2014, 136, 7869).
example 2
[0121] 1b[( iPr PNHP)Fe(H)(CO)(HBH 3 )] improved synthesis of
[0122]
[0123] In a glove box, under a nitrogen atmosphere, a 200-mL oven-dried Schlenk flask was charged with the complex [ iPr PNHP]FeBr 2 (CO) (850mg, 1.545mmol), NaBH 4 (131 mg, 3.399 mmol, 98% purity) and 100 mL of dry EtOH. The resulting yellow solution was stirred at room temperature for 18 hours and filtered through Celite. The filtrate was evaporated to dryness to afford pure 1b (isolated yield 84%). 1b 1 H and 31 P{ 1 H} NMR spectra are in good agreement with reported values (see S. Chakraborty et al., J. Am. Chem. Soc. 2014, 136, 7869).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com