Method for preparing hexamethylenediamine from adipic dialdehyde based on Ni-based catalyst
A catalyst, adipaldehyde technology, which is applied in reductive alkylation preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of dependence on imports, high price, high toxicity of adiponitrile, etc., and achieves low cost , easy to operate, reduce the loss of metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
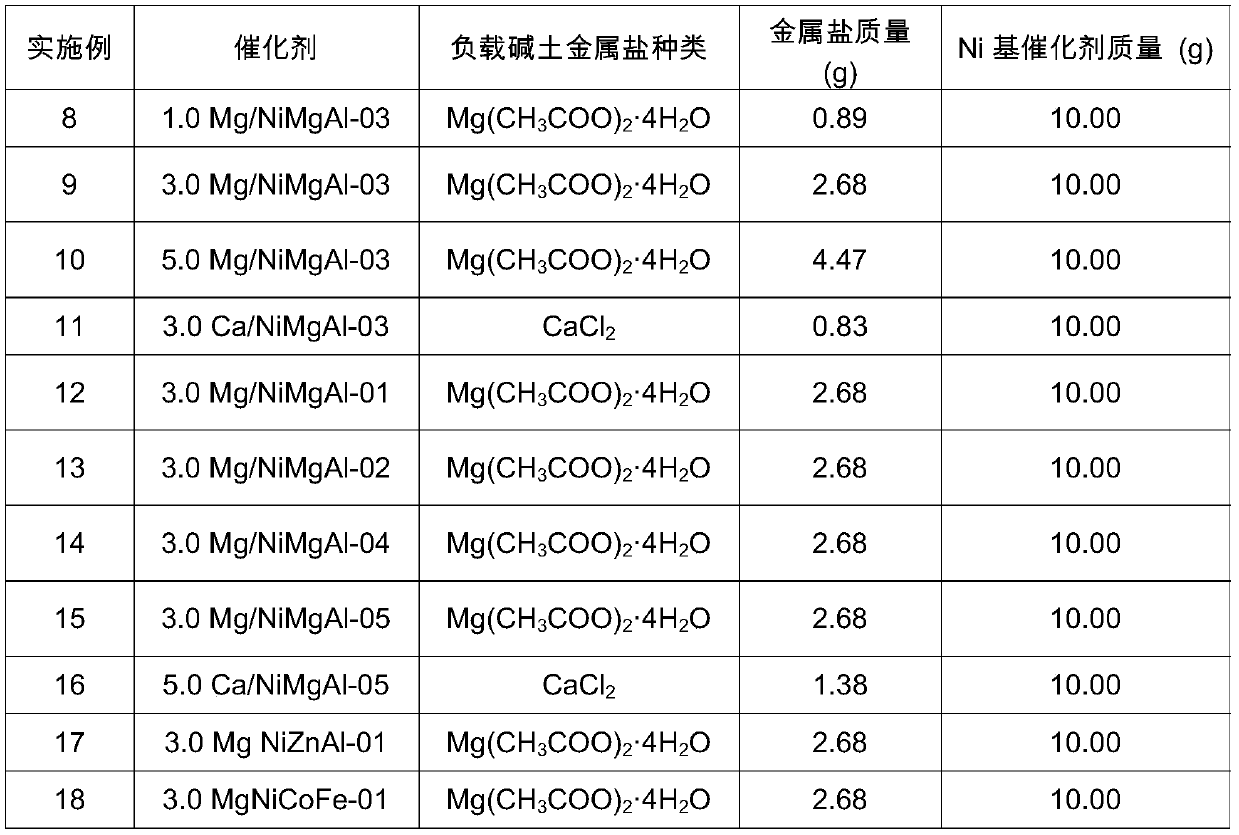
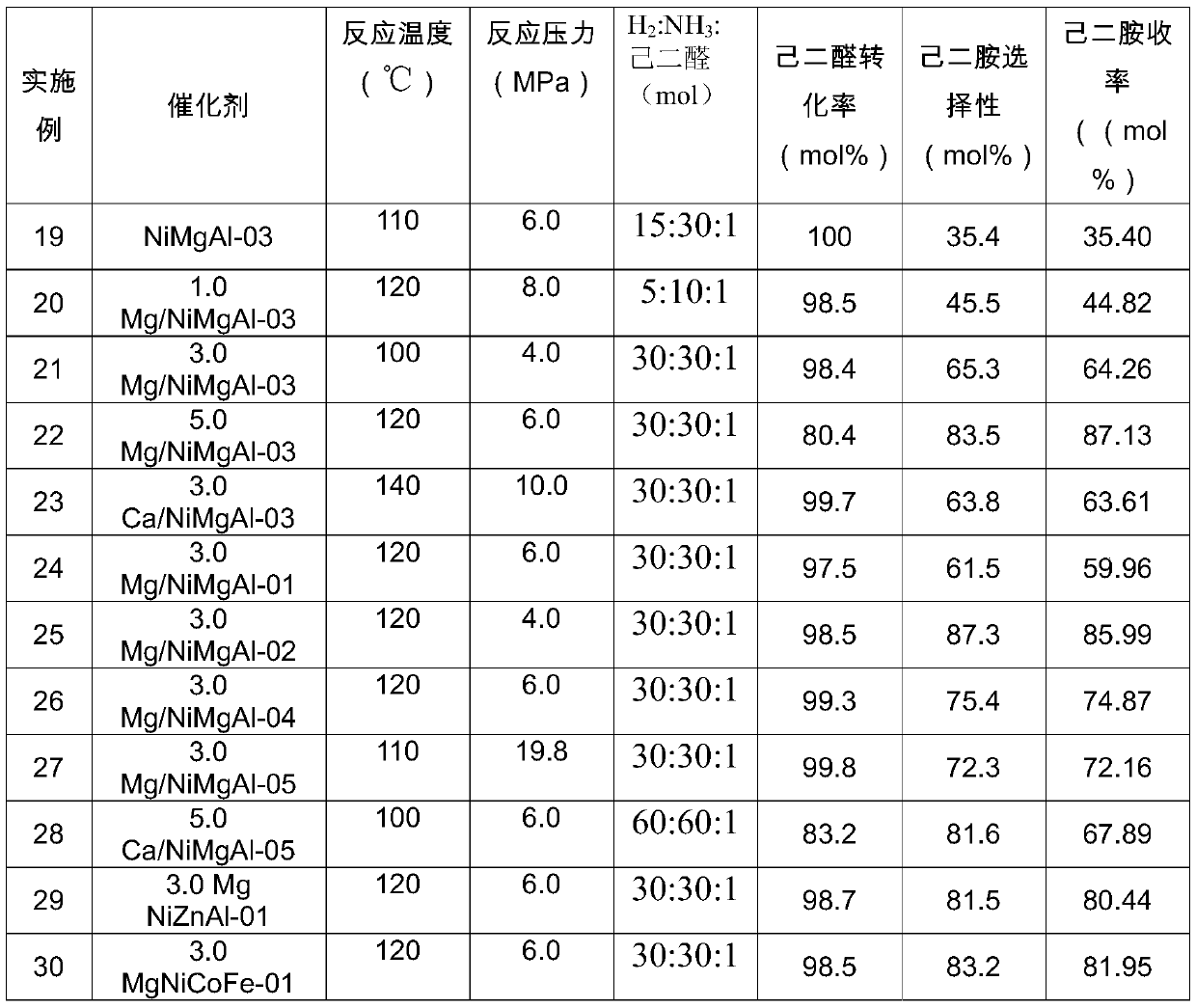
[0055] Embodiment 1 prepares NiMgAl-01 catalyst with hydrotalcite as precursor, (Ni 2+ +Mg 2+ ):Al 3+ The ratio is 3:1, Ni 2+ : Mg 2+The ratio is 0.1.
[0056] 9.52gNi(NO 3 ) 2 ·6H 2 O, 46.89gAl(NO 3 ) 3 9H 2 O and 83.92gMg(NO 3 ) 2 ·6H 2 O is completely dissolved in 500ml of water to obtain mixed solution I; weigh 26.5gNa 2 CO 3 and 70g of NaOH were completely dissolved in 1L of water to obtain the mixed solution II; the two mixed solutions were slowly added dropwise to 200mL of water at the same time, vigorously stirred at a speed of 600r / min, and the pH was maintained at 10. After the addition was completed, the obtained colloidal suspension Transfer to an oven, age at 65°C for 72h, wash with distilled water until neutral, and dry to obtain Ni-based hydrotalcite, and further roast the obtained Ni-based hydrotalcite precursor at 500°C for 4h to obtain highly dispersed Ni base catalyst.
Embodiment 2
[0057] Embodiment 2 prepares NiMgAl-02 catalyst with hydrotalcite as precursor, (Ni 2+ +Mg 2+ ):Al 3+ The ratio is 3:1, Ni 2+ : Mg 2+ The ratio is 1.0.
[0058] 52.34gNi(NO 3 ) 2 ·6H 2 O, 46.89gAl(NO 3 ) 3 9H 2 O and 46.15gMg(NO 3 ) 2 ·6H 2 O is completely dissolved in 500ml of water to obtain mixed solution I; weigh 26.5gNa 2 CO 3 and 70g of NaOH were completely dissolved in 1L of water to obtain the mixed solution II; the two mixed solutions were slowly added dropwise to 200mL of water at the same time, vigorously stirred at a speed of 600r / min, and the pH was maintained at 10. After the addition was completed, the obtained colloidal suspension Transfer to an oven, age at 65°C for 72h, wash with distilled water until neutral, and dry to obtain Ni-based hydrotalcite, and further roast the obtained Ni-based hydrotalcite precursor at 500°C for 4h to obtain highly dispersed Ni base catalyst.
Embodiment 3
[0059] Embodiment 3 prepares NiMgAl-03 catalyst with hydrotalcite as precursor, (Ni 2+ +Mg 2+ ):Al 3+ The ratio is 3:1, Ni 2+ : Mg 2+ The ratio is 3.0
[0060] 78.52gNi(NO 3 ) 2 ·6H 2 O, 46.89gAl(NO 3 ) 3 9H 2 O and 23.07gMg(NO 3 ) 2 ·6H 2 O is completely dissolved in 500ml of water to obtain mixed solution I; weigh 26.5gNa 2 CO 3 and 70g of NaOH were completely dissolved in 1L of water to obtain the mixed solution II; the two mixed solutions were slowly added dropwise to 200mL of water at the same time, vigorously stirred at a speed of 600r / min, and the pH was maintained at 10. After the addition was completed, the obtained colloidal suspension Transfer to an oven, age at 65°C for 72h, wash with distilled water until neutral, and dry to obtain Ni-based hydrotalcite, and further roast the obtained Ni-based hydrotalcite precursor at 500°C for 4h to obtain highly dispersed Ni base catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com