Nucleic acid molecules and uses thereof
A nucleic acid molecule and promoter technology, which can be applied to factors such as factor VII, coagulation/fibrinolysis factor, and the introduction of foreign genetic material using vectors, which can solve problems such as hindering repeated treatment of AAV.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0520] V. Preparation of Polypeptides
[0521] The present disclosure also provides polypeptides encoded by the nucleic acid molecules of the present disclosure. In other embodiments, the polypeptides of the present disclosure are encoded by vectors comprising the nucleic acid molecules of the present disclosure. In yet other embodiments, the polypeptides of the present disclosure are produced by host cells comprising the nucleic acid molecules of the present disclosure.
[0522] In other embodiments, the present disclosure also provides methods of producing a polypeptide having coagulation factor (eg, FVIII) activity, comprising culturing a host cell of the present disclosure under conditions that produce a polypeptide having coagulation factor (eg, FVIII) activity, And the polypeptide with coagulation factor (eg, FVIII) activity is recovered. In some embodiments, the expression of a polypeptide having coagulation factor (eg, FVIII) activity is relative to a host cultured u...
Embodiment 1
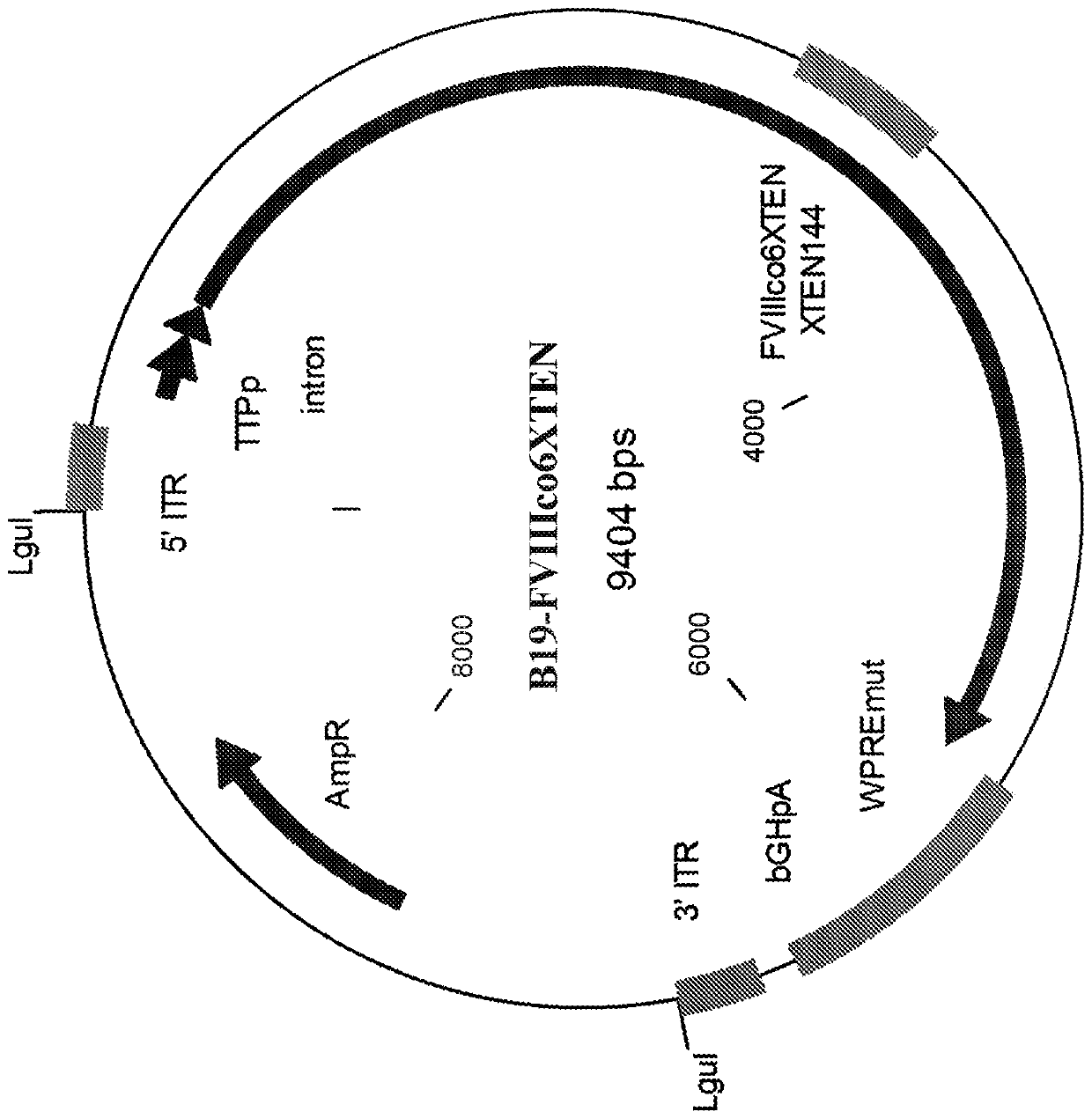
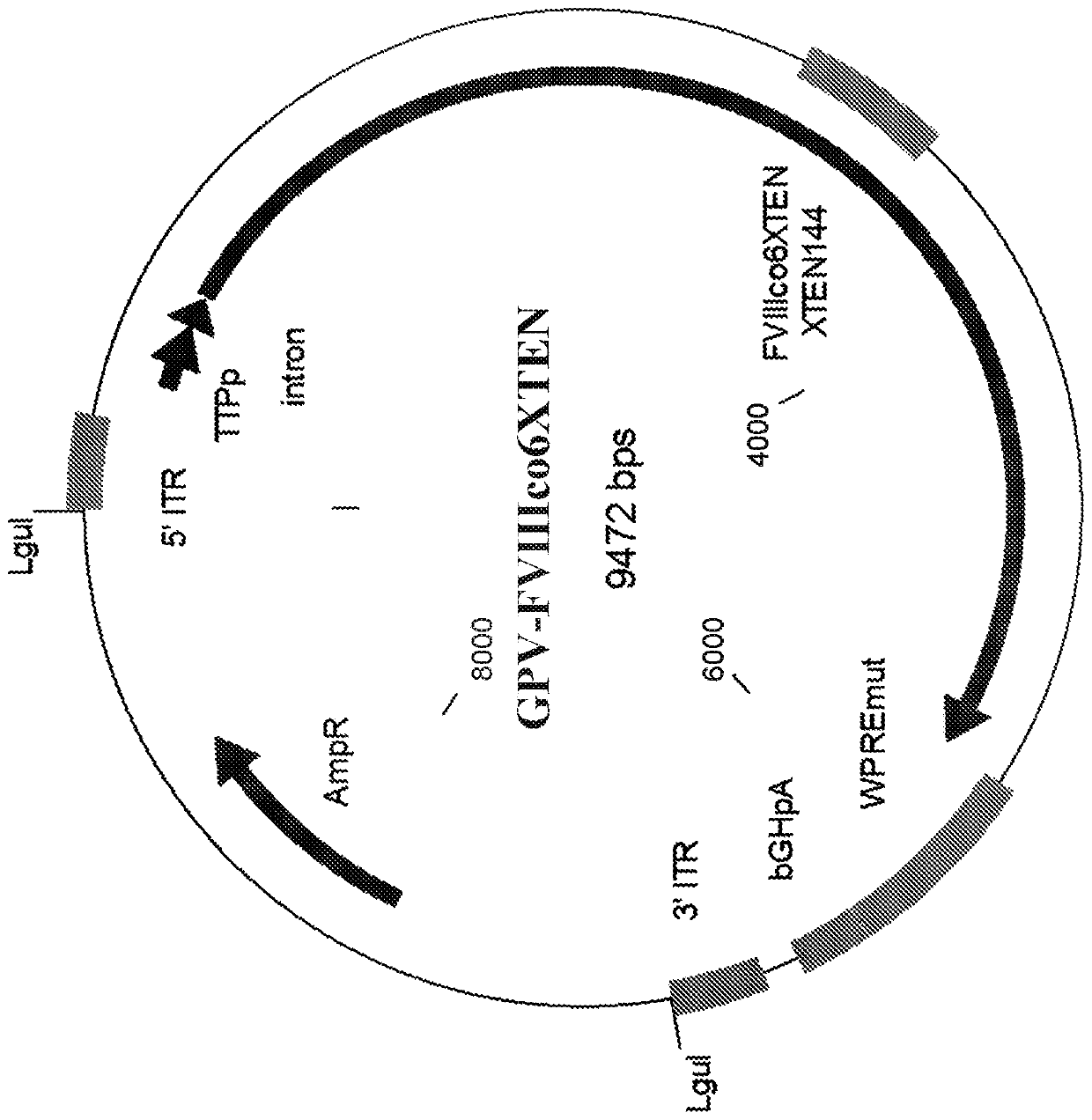
[0590] Example 1. Generation of FVIII expression constructs with AAV and non-AAV parvoviral ITRs.
Embodiment 1a
[0591] Example 1a. Cloning of the codon-optimized FVIII gene and the inverted terminal repeat (ITR) region from AAV into a gene cassette.
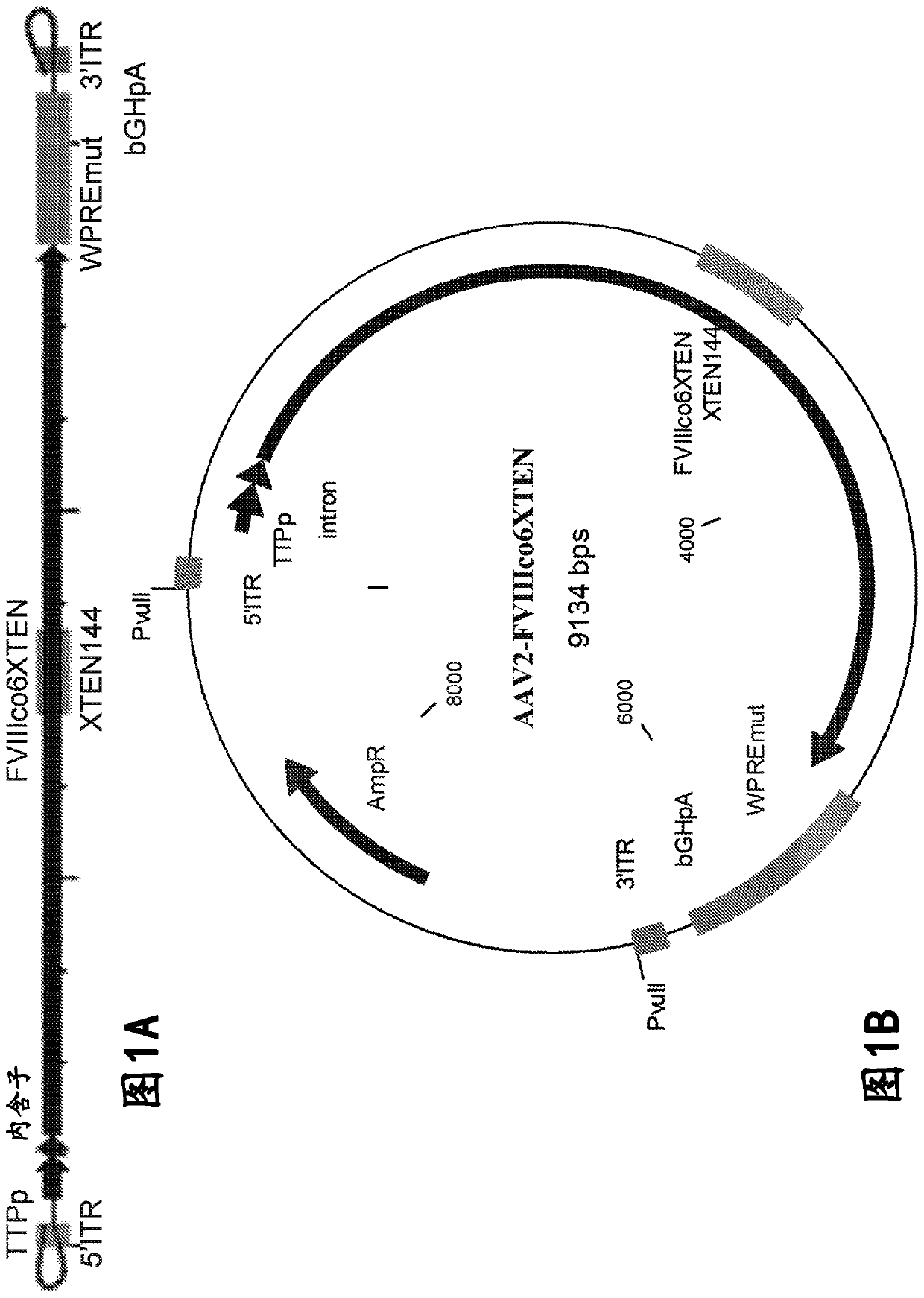
[0592] The FVIII gene cassette was generated based on the genome of AAV serotype 2. However, ITR regions derived from any serotype (including synthetic) can be used in this method ( Figure 1A ).
[0593] An expression plasmid AAV2-FVIIIco6XTEN encoding a codon-optimized FVIII coding sequence was designed for in vitro and in vivo expression, flanked by a liver-specific promoter from the inverted terminal repeat (ITR) region of AAV (AAV-FVIII) (TTPp, Figure 1A and 1B ) under the control of Figure 1B shown in. The gene cassette also contains WPRE and bGHpA elements for optimal expression of the transgene ( Figure 1A and 1B ). The codon-optimized FVIII sequence flanked by ITR was cloned into a plasmid backbone containing the ColE1 origin of replication and the expression cassette for the beta-lactamase that confers ampicillin resistan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com