Ciclosporin composition for treating hemolytic anemia and application of composition
A technology for hemolytic anemia and a composition, applied in the field of medicine, can solve the problems of strong adverse reaction effect, difficult long-term tolerance of patients, etc., achieves low side effects and adverse reaction rates, and increases safety, compliance, side effects and adverse reactions. rate reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
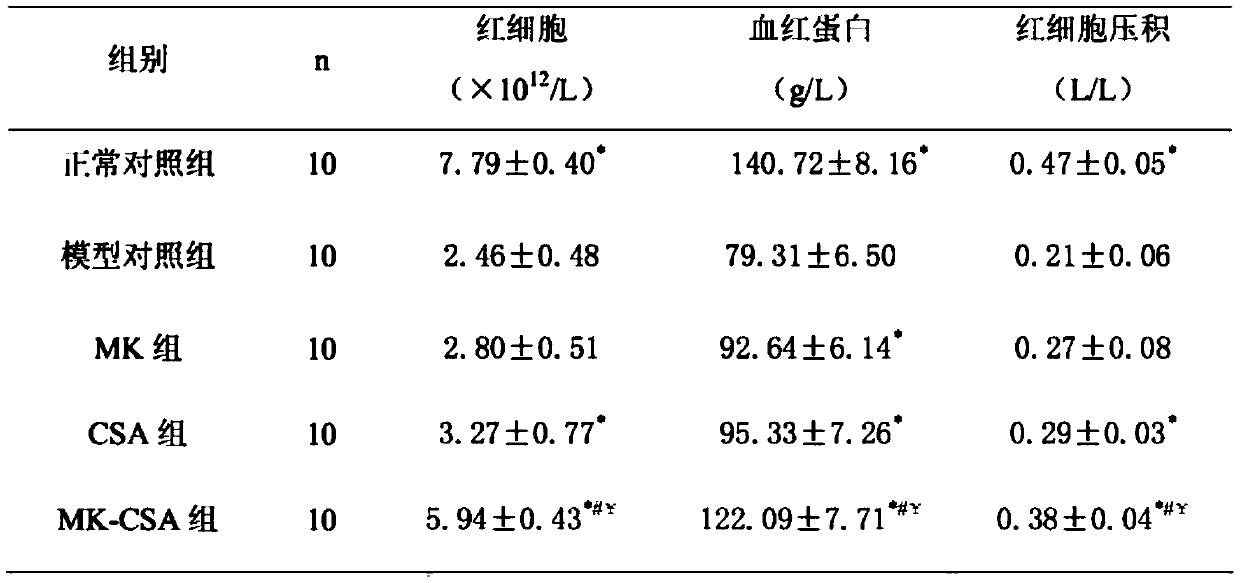
[0015] Example 1 Efficacy test of montelukast sodium combined with cyclosporine on hemolytic anemia model rats
[0016] 50 Wistar rats, weighing 180-220 g. Breeding conditions: room temperature at 20-25°C, relative humidity at 38%-41%, artificial lighting, light and dark for 12 hours, solid common feed and tap water, free intake of food and water, good ventilation; clean indoor sanitation twice a day, keep indoor No obvious ammonia odor.
[0017] 50 rats were randomly divided into the following five groups: normal control group, model control group, MK group, CSA group, MK-CSA group, 10 rats in each group, half male and half male. After the rats were fed adaptively for one week, except the normal control group, the other groups were intraperitoneally injected with 2% acetylphenylhydrazine normal saline solution 0.2g / kg on the first day and the fourth day respectively, and the normal control group was injected with normal saline instead. On the same day after successful model...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com