Method for quantitatively detecting human T-cell lymphotropic virus provirus
A technology for quantitative detection of lymphocytes, applied in the field of medical testing, can solve the problems of time-consuming, laborious, and inability to accurately quantify the number of virus copies, and achieve high sensitivity, high repeatability, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Establishment of plasmid standards
[0058] 1. Materials
[0059] 1. Restriction endonuclease and buffer: American NEB company
[0060] 2. Hut102 cells: purchased from Peking Union Medical College
[0061] 3. NanoDrop 2000 micro-volume ultraviolet spectrophotometer: American Thermo Company
[0062] 4. Plasmid extraction kit: Axygen, USA
[0063] 5. T4 ligase and buffer: American NEB company
[0064] 6. Ordinary DNA gel recovery kit: Tiangen Biochemical (Beijing) Technology Co., Ltd.
[0065] 7. pcDNA3.1(-) plasmid: purchased from Invitrogen
[0066] 8. Competent cells of Escherichia coli DH5α: Beijing Qingke Biotechnology Co., Ltd.
[0067] 9. PCR amplification buffer (gold medal mix): Beijing Qingke Biotechnology Co., Ltd.
[0068] 2. Method
[0069] 1. Amplify the HTLV Pol gene sequence
[0070] HTLV Pol upstream and downstream primers (5' cagtagactagtccaaaccctgcccctccta (SEQ ID NO.7) and 5' tggcgagaaacttacccatggtgttggtg (SEQ ID NO.8)) were applied...
Embodiment 2
[0130] Embodiment 2: Quantitative detection of HTLV-1 positive cell Hut102 cell copy number
[0131] 1. Materials
[0132] 1. Magnetic Bead Method Blood Genomic DNA Extraction Kit (Model: DP341): Tiangen Biochemical Technology (Beijing) Co., Ltd.
[0133] 2. Hut102 cells: purchased from Peking Union Medical College
[0134] 3. ABI7500 real-time quantitative fluorescent PCR instrument: Applied Biosystems, USA
[0135] 4.qPCR 5×Hyperstart Pre-mix: Zhuhai Baorui Biological Co., Ltd.
[0136] 2. Method
[0137] 1. Extraction of Genomic DNA
[0138] The blood genomic DNA extraction kit (model: DP341) produced by Beijing Tiangen Company was used to extract, and the gDNA of Hut102 cells (purchased by Peking Union Medical College) was extracted according to the instructions.
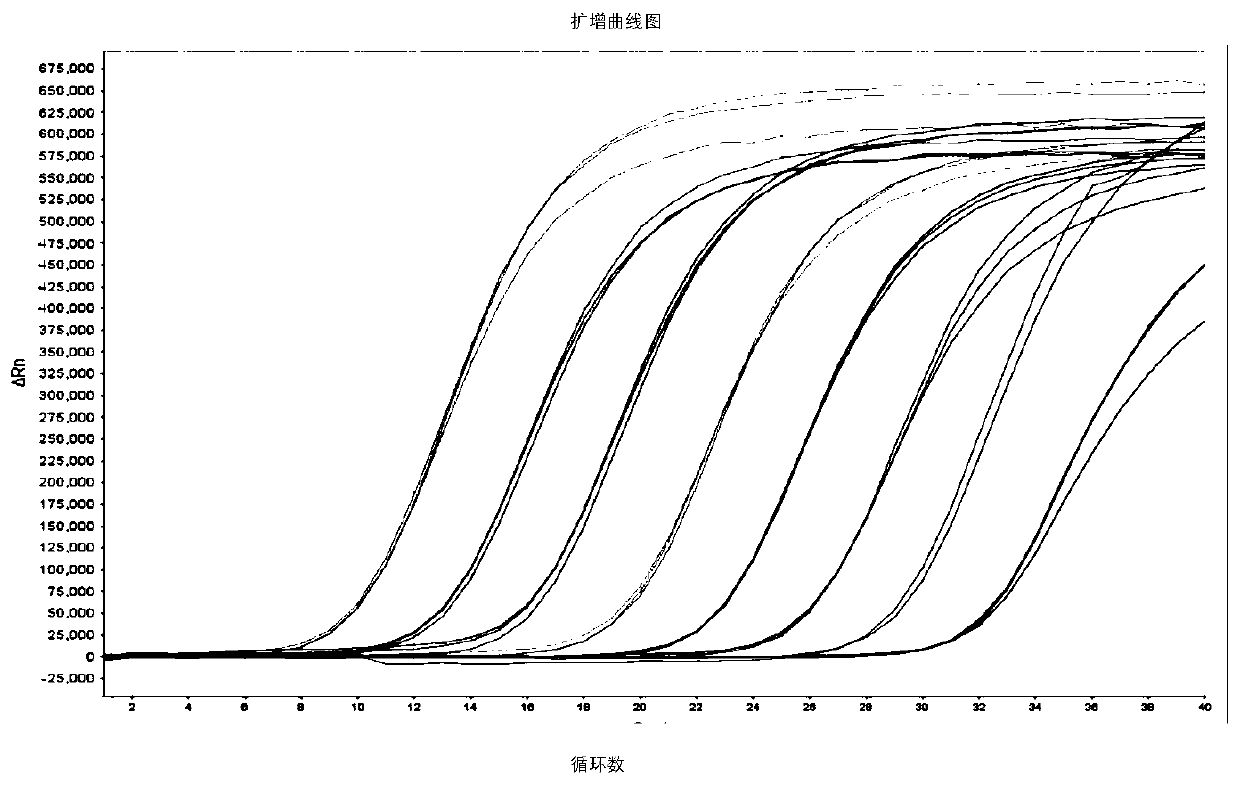
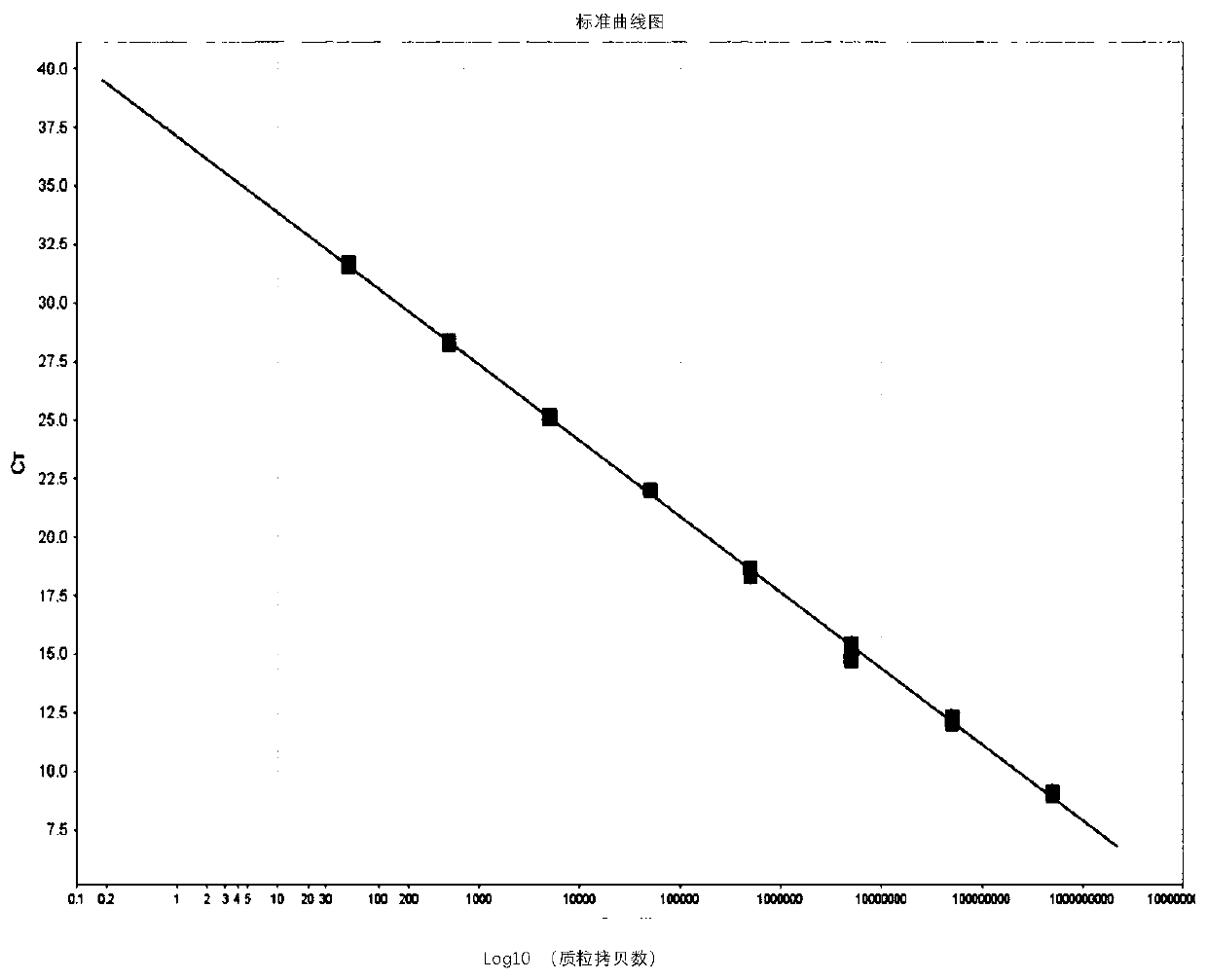
[0139] 2. Establish a standard curve
[0140] Choose 5×10 8 copies / μL~5×10 1 Copies / μL gradient plasmid standard to establish a standard curve: each concentration of the plasmid standard is set up 3 para...
Embodiment 3
[0150] Example 3: Quantitative detection of HTLV-1 positive proviral load in 3 cases confirmed by INNO-LIA and WB
[0151] 1. Materials
[0152] 1. Magnetic Bead Method Blood Genomic DNA Extraction Kit (Model: DP341): Tiangen Biochemical Technology (Beijing) has
[0153] limited company
[0154] 2. Hut102 cells: purchased from Peking Union Medical College
[0155] 3. ABI7500 real-time quantitative fluorescent PCR instrument: Applied Biosystems, USA
[0156] 4.qPCR 5×Hyperstart Pre-mix: Zhuhai Baorui Biological Co., Ltd.
[0157] 2. Method
[0158] 1. Extraction of sample genomic DNA
[0159] The blood genomic DNA extraction kit produced by Beijing Tiangen Company was used for extraction, and the gDNA in the sample to be tested was extracted according to the instructions.
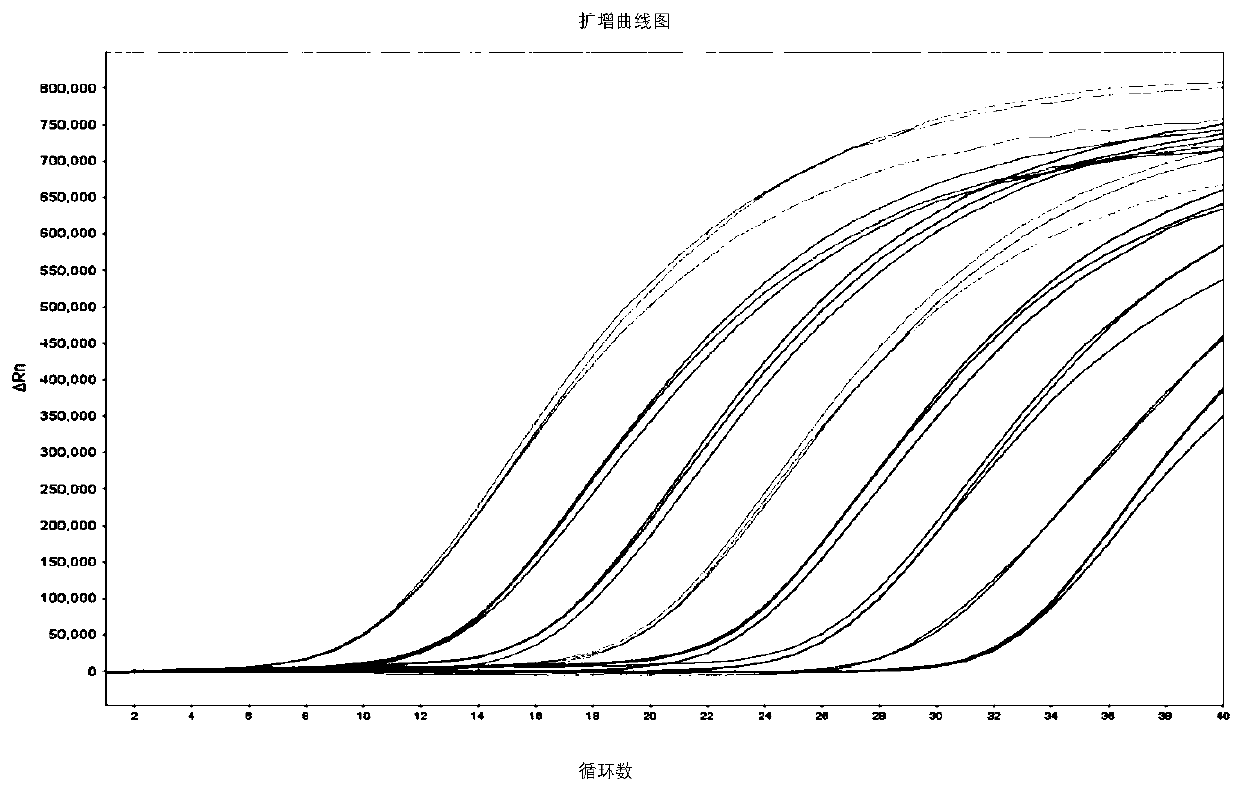
[0160] 2. Make a standard curve
[0161] Choose 5×10 6 copies / μL~5×10 2 Copies / μL gradient plasmid standards were used to establish a standard curve: 3 parallel reactions were set up for each concen...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap