Pharmaceutical composition for treating laryngeal cancer, its preparation method and application
A technology of composition and medicine, which is applied in the field of pharmaceutical composition for the treatment of laryngeal cancer, can solve the problems of few reports on pharmacological effects, and achieve good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The present embodiment provides a pharmaceutical composition, the active ingredients of which include the biflavones of Spiraea spiconiae, Ginkgo biflavones, Cypress biflavones, Bamboo biloba biflavones A, 7-desmethyl Ginkgo biflavones and 7,4',7",4 ”'-Tetramethoxy-Tetramethoxy-Taquinone, and the mass ratio is 52.7:7.8:4.7:2.3:8.2:2.5.
[0082] The embodiment of the present invention also provides a preparation method of the above-mentioned pharmaceutical composition, comprising:
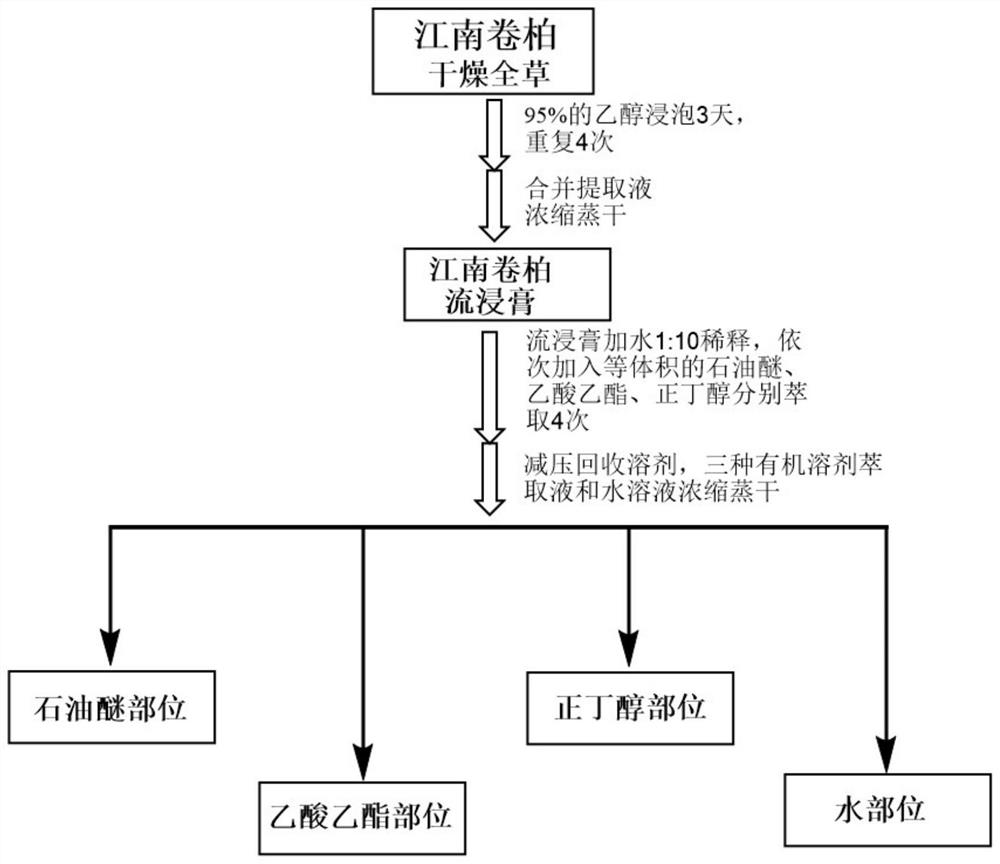
[0083] refer to figure 1 The process flow shown is to extract Selaginella jiangnan to obtain a pharmaceutical composition containing the above active ingredients.
[0084] Specifically, the dried whole herb of Selaginella jiangnanensis (500 g) was ground, then immersed in 95% EtOH four times (2.5 L each time, 3 days) at room temperature for extraction. The solvent was evaporated under reduced pressure to obtain a crude extract (61.5 g). The crude extract was suspended in water by adding wa...
experiment example
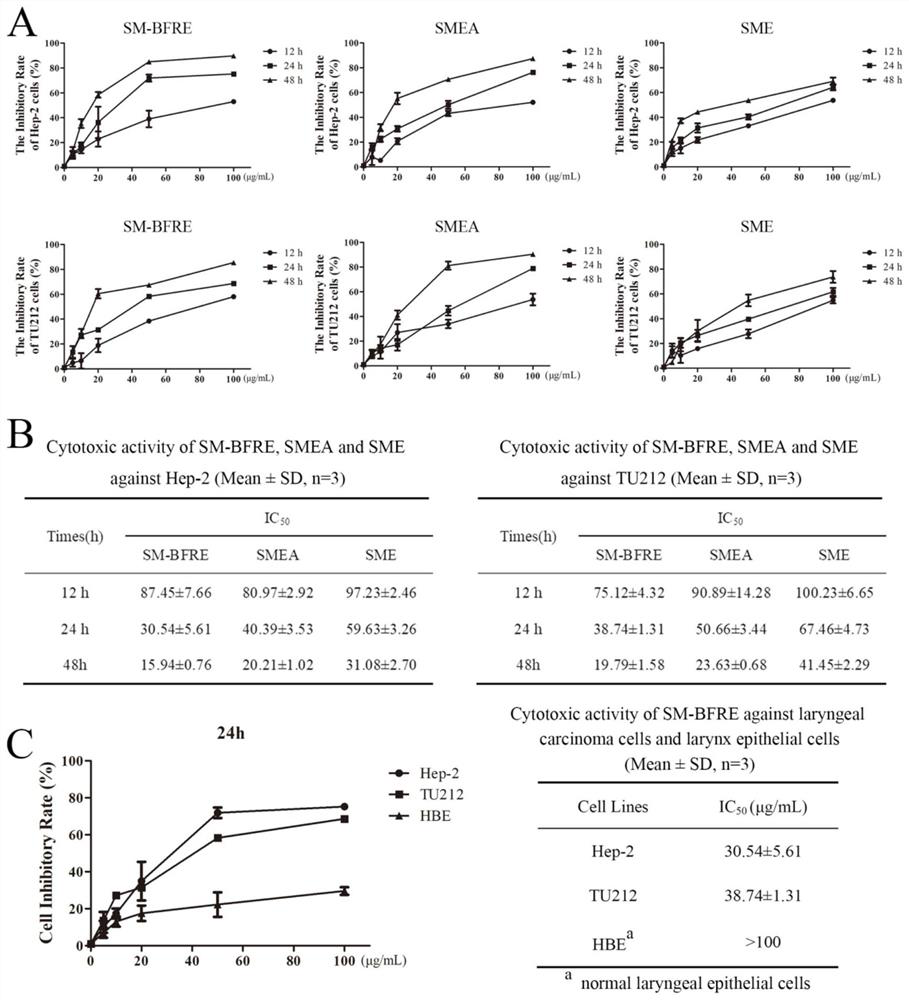
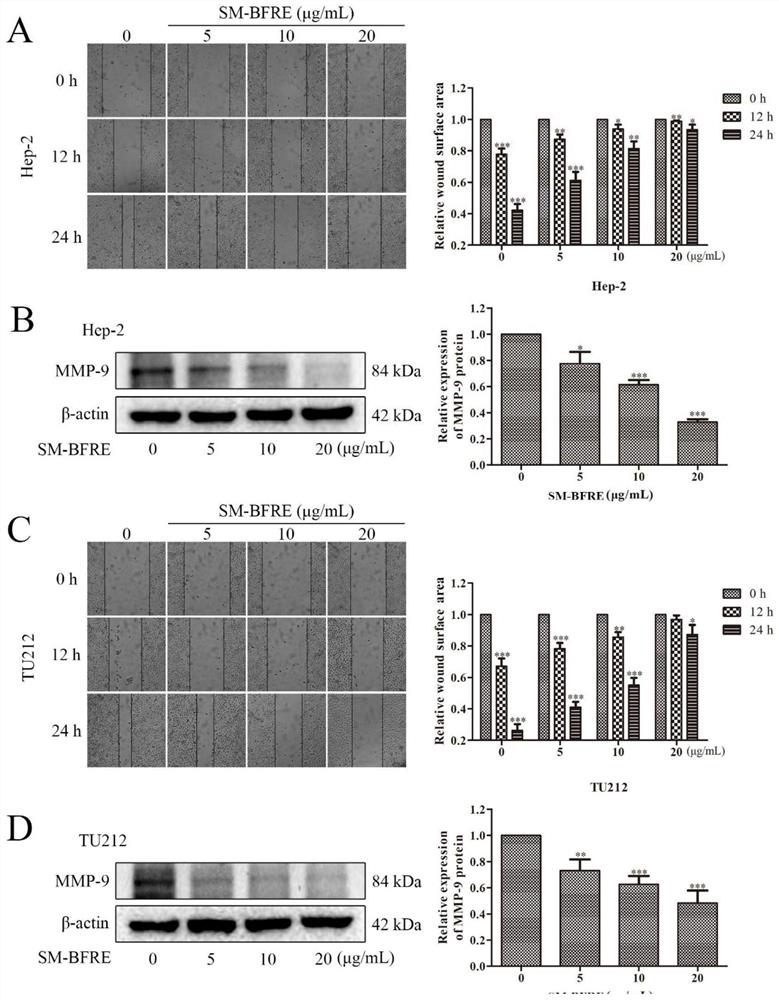
[0088] The drug efficacy evaluation of the Selaginella chinensis biflavonoid enrichment provided in Example 1 of the present invention will be conducted below in combination with the drug efficacy test.
[0089] sample:
[0090] Sample to be tested: the Selaginella chinensis biflavone enrichment provided in Example 1;
experiment example 1
[0093] Experimental example 1, detection of the effect of the test article, comparative examples 1 and 2 on the viability of human laryngeal cancer cells Hep-2 and TU212 and normal laryngeal epithelial cells HBE cells, and the IC 50 value
[0094] Take Hep-2 and TU212 cell lines in the logarithmic growth phase, and adjust the cell concentration to 5×10 4 1 / mL, 0.1mL per well was inoculated into a 96-well culture plate, and after continuing to cultivate for 24h, each well was added with 0.2mL of the sample to be tested prepared by DMEM solution containing 10% calf serum, so that the sample concentration was 5 μg / mL , 10 μg / mL, 20 μg / mL, 50 μg / mL and 100 μg / mL for 12 h, 24 h and 48 h; each concentration was replicated in 3 wells, and the experimental control without test samples and the blank control well without samples and cells were set up . Add 0.1mL MTT to each well and continue to incubate for 30min after the dosing time is reached. Discard the culture medium after the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com