Kit for detecting congenital aniridia pathogenic gene mutation and application of kit
A technology for detecting kits and disease-causing genes, which is applied in the field of preparation of genetic diagnostic products to achieve the effects of simple operation, low cost and direct results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1 congenital aniridia pathogenic gene mutation detection kit
[0020] 1. Design and synthesis of primer pairs
[0021] Primer 1: ATAGCAGGGAACTGACCGCC (shown in SEQ ID NO: 1)
[0022] Primer 2: TGTAACTGACCCAGGTTGAAAGAGA (shown in SEQ ID NO: 2)
[0023] It was synthesized by an automatic DNA synthesizer and diluted to 10 pmol / L.
[0024] 2. Assembling the kit
[0025] Including: 10×PCR reaction buffer, 25mM dNTP, DNA polymerase, PCR amplification primer pair and ddH 2 O.
Embodiment 2
[0026] Example 2 Application of Congenital Aniridia Pathogenic Gene Mutation Detection Kit
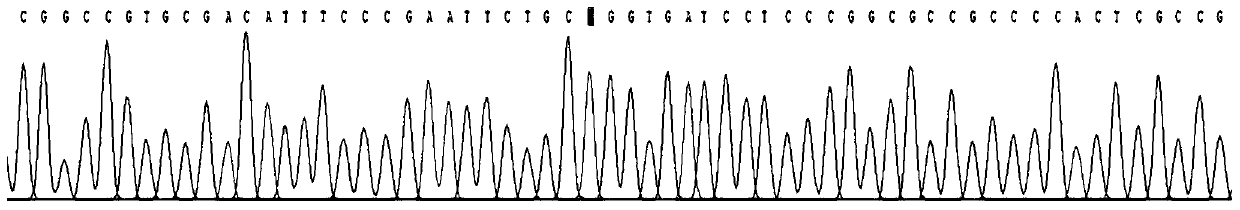
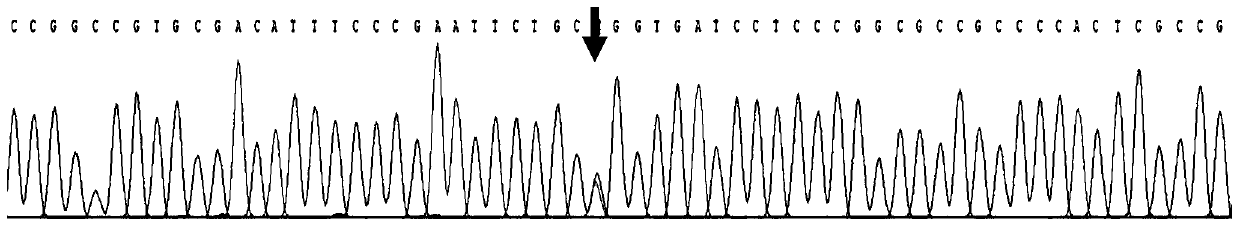
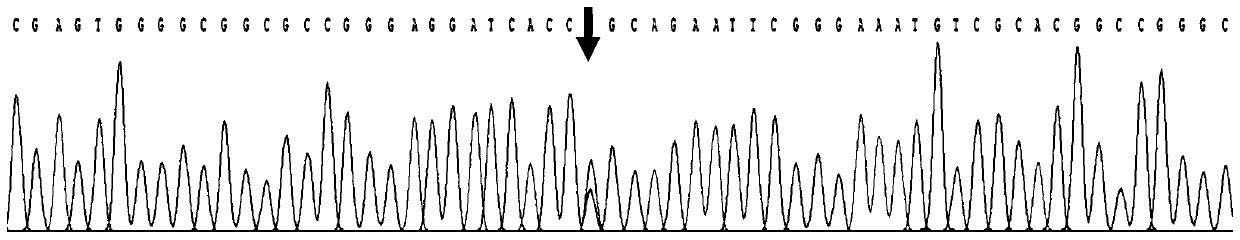
[0027] Use the kit obtained in Example 1 to amplify the PAX6 gene on the genomic DNA of the subject to be tested. After PCR, the product is subjected to Sanger sequencing analysis and the sequencing results are read.
[0028] 1. The specific steps are as follows:
[0029] (1) Extract the genomic DNA of the subject to be tested and dilute it to 100-200ng / ul.
[0030] (2) Synthetic primers:
[0031] Primer 1: ATAGCAGGGAACTGACCGCC (shown in SEQ ID NO: 1)
[0032] Primer 2: TGTAACTGACCCAGGTTGAAAGAGA (shown in SEQ ID NO: 2)
[0033] (3) In vitro amplification (PCR) of sample DNA target fragment
[0034] The PCR reaction system is 50ul, and the specific components are as shown in Table 1 below:
[0035] Table 1 PCR reaction system
[0036]
[0037]
[0038] After the above-mentioned mixed liquid was mixed, the target fragment was amplified on the PCR instrument, and the specific ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com