Fusion-proteins based on human ferritin and protease-cleavable peptides and their use as chemotherapeutics carriers
A technology of fusion protein and protease, applied in the field of fusion protein, can solve problems such as decreased mineralization, osteopenia, and inhibition of human osteoblasts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
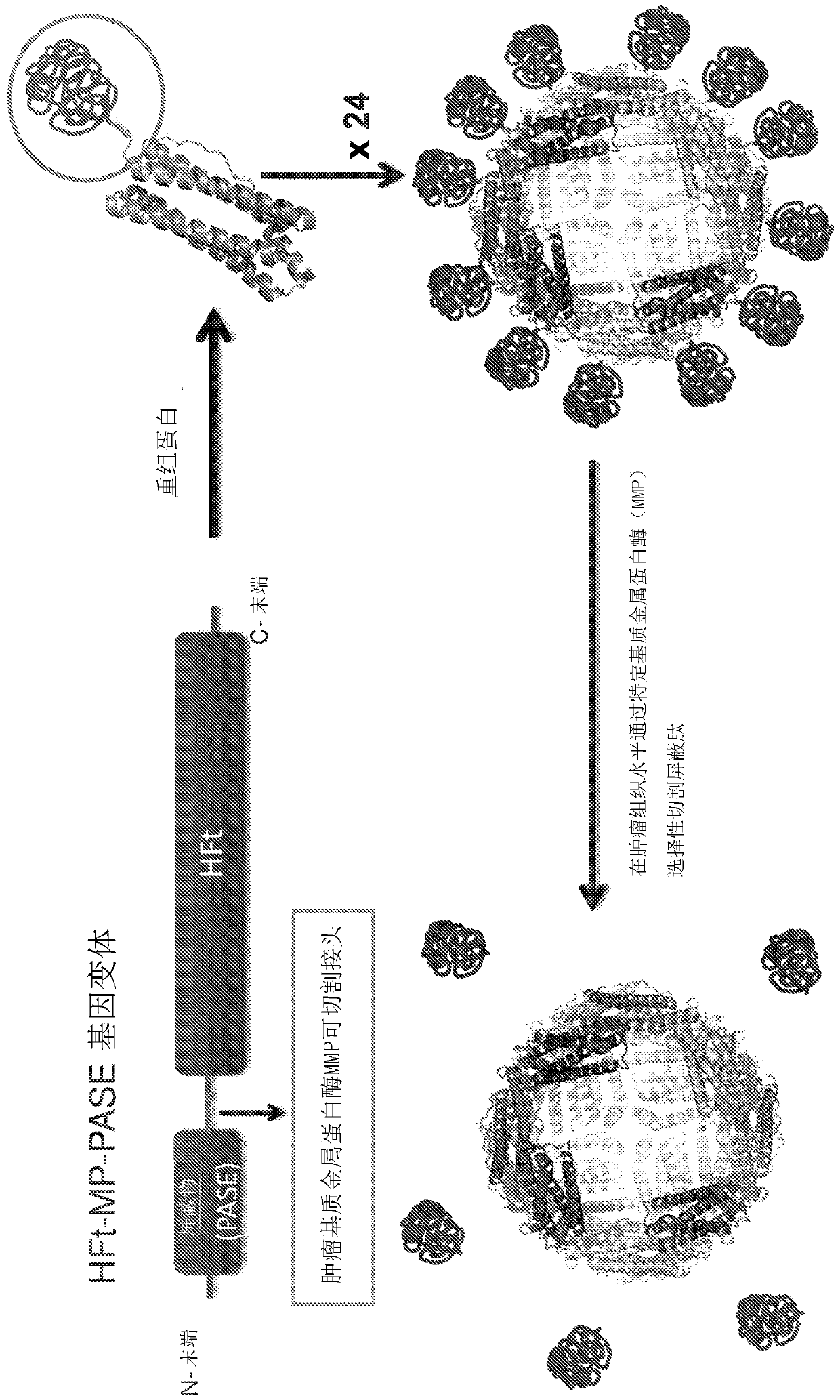
[0075] Example 1 is used for the construction of the expression vector of HFt-MP-PASE fusion protein
[0076] To reduce protein aggregation and increase stability, it was decided to introduce negatively charged residues in the outer shield-forming PAS sequence fused to each HFt subunit. Analysis of previously obtained HFt-MP-PAS constructs did show that the introduction of two glutamic acid residues in the 40-residue PAS sequence induces sufficient electrostatic repulsion to prevent dissimilar 24-mer assemblies from forming between each other. aggregation without affecting subunit assembly within the 24-mer. In order to distribute as much negative charge as possible on the surface of the construct, glutamic acid residues were placed at a distance of 20 residues.
[0077] The HFt-MP-PASE gene was obtained by combining three different sequences into one single sequence: HFt (SEQ ID NO: 1), MP (SEQ ID NO: 5) and PASE (SEQ ID NO: 10). This construct differs from the previously p...
example 2
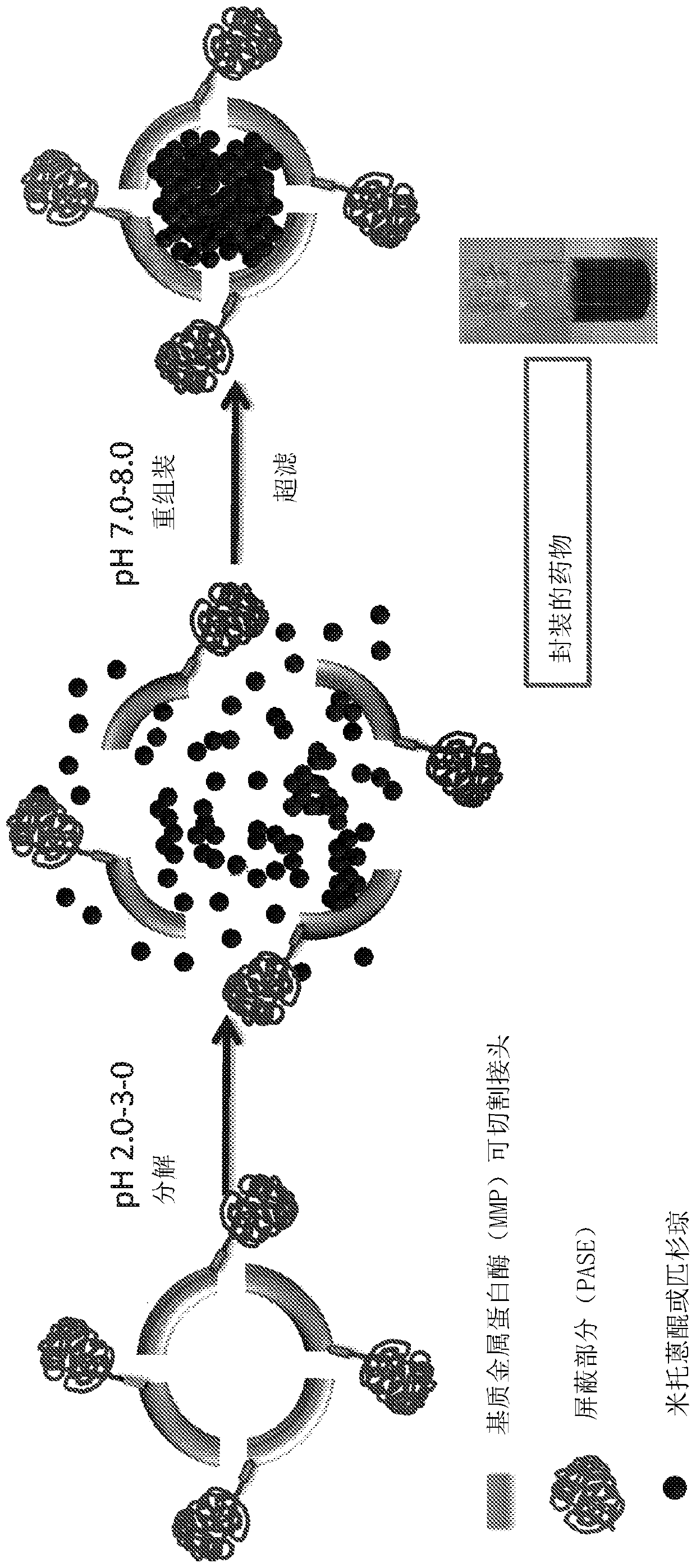
[0080] Example 2 The preparation of HFt-MP-PAS and HFt-MP-PASE carrying chemotherapeutic drugs
[0081] As a chemotherapeutic agent, the inventors reported an example in which the drug mitoxantrone (MIT) or picantron (PIX) was used. According to the protocol disclosed in Falvo et al., 2016, Biomacromolecules. 17(2):514 22, these drugs were encapsulated within the protein cavity of the fusion protein by utilizing the coupling process of protein uncoupling as a function of pH. Disassembly / reassembly process such as image 3 shown.
example 3
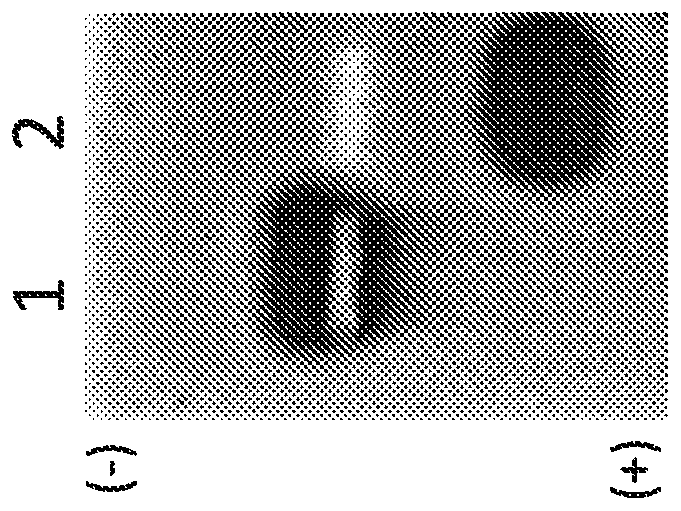
[0082] Example 3 Test the encapsulation yield of drug mitoxantrone and picantron
[0083] The encapsulation efficiency of MIT or PIX in HFt-MP-PASE was compared with that in native HFt and HFt MP PAS ( Figure 4 ). In all experiments performed, HFt-MP-PASE outperformed both native HFt and HFt-MP-PAS proteins in terms of the amount of protein recovered at the end of the reaction. This indicates that HFt-MP-PASE is more stable and does not aggregate / precipitate out of solution during protein-drug complex formation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com