Pyroxasulfone synthesis method
A synthesis method and a technology for sulfofenazone are applied in the field of organic synthesis, and can solve the problems of increased product cost, production safety, unpleasant smell of compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
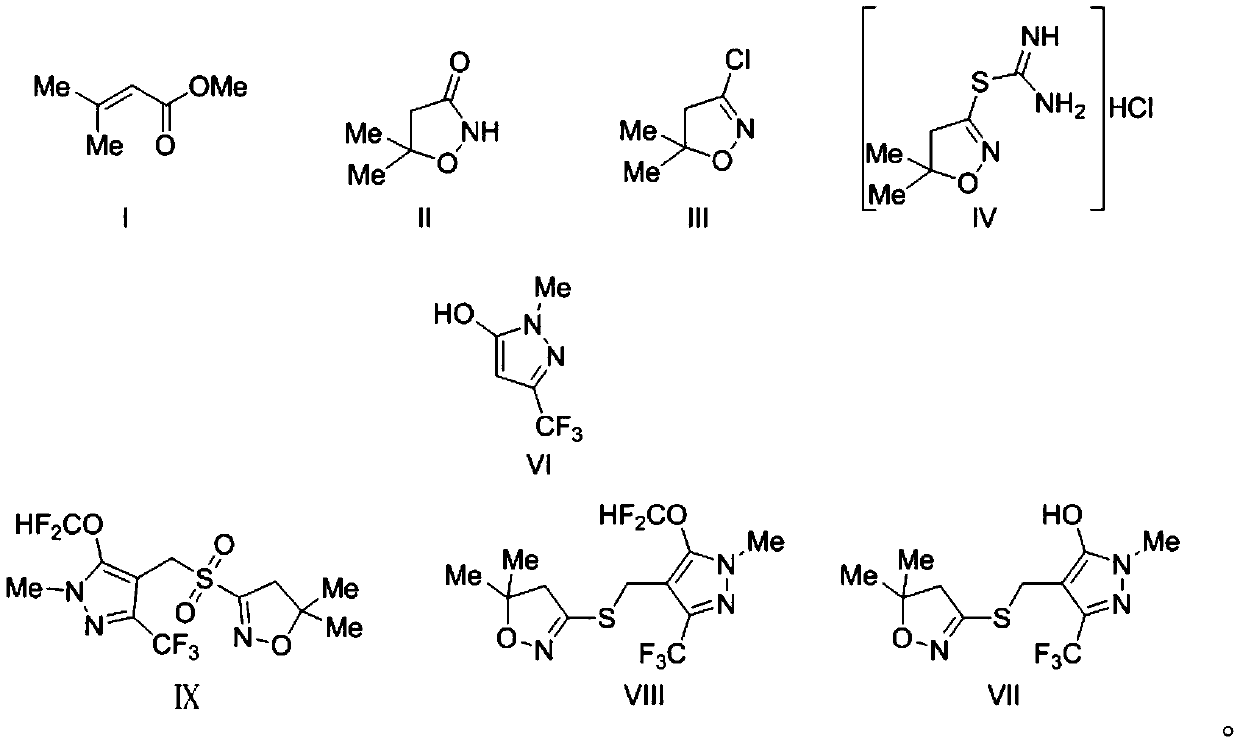
[0031] The invention provides a kind of synthetic method of pyrazafen, comprising the following steps:
[0032] Using compound I as the starting material, the intermediate II is synthesized through a cyclization reaction, and the intermediate II is subjected to a chlorination reaction under the action of a chlorinating agent to obtain the intermediate III; the intermediate III is reacted with thiourea to obtain the salt Salt intermediate IV;
[0033] The chlorination reagent is phosphorus pentachloride and / or phosphorus oxychloride;
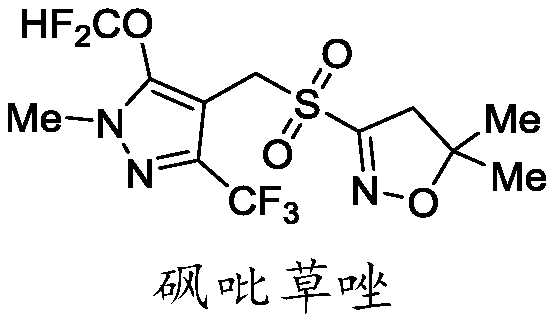
[0034] The hydrochloride intermediate IV, compound VI react with formaldehyde to obtain intermediate VII, and the intermediate VII undergoes a difluoromethoxylation reaction under the condition of a catalyst to obtain intermediate VIII, and finally use hydrogen peroxide to treat The intermediate VIII is oxidized to obtain pyroxazole IX;
[0035] Described catalyst is sodium tungstate and acid;
[0036] The synthetic route among the present inv...
Embodiment 1
[0079] (1) Synthesis of compound II
[0080] Under the condition of nitrogen protection, 83.6 g (1.1 mol) of hydroxyurea was added into 500 mL methanol solution (2.4 mol / L) of potassium methoxide, and then stirred and dissolved at 25°C. At the same temperature, 128 g (1 mol) of ethyl 3,3-dimethacrylate was added dropwise for half an hour. Then it was stirred at 25°C for 18 hours. After the reaction was completed, the reaction solution was filtered to obtain a white solid, which was then dissolved in water and neutralized by adding 100 mL of hydrochloric acid (35%, 1.2 mol). Finally, it was extracted three times with 200 mL of 1,2-dichloroethane, the organic phases were combined, dried, and the solvent was distilled off under reduced pressure to obtain 91 g of a white solid with a yield of 80%.
[0081] (2) Synthesis of compound III
[0082] At 25°C, under nitrogen protection conditions, 99.5g of phosphorus pentachloride (0.48mol) was dissolved in 100mL of 1,2-dichloroethane...
Embodiment 2
[0095] Step (2) The synthesis of compound III was carried out according to the following steps, and the rest of the steps were carried out according to the method in Example 1.
[0096] (2) Synthesis of compound III
[0097] At 25°C, under nitrogen protection, 50g of phosphorus pentachloride (0.24mol) was dissolved in 100mL of toluene, compound II (25g, 0.22mol) was added to the solution in batches, and stirred at 25°C for 5 Hours. Control the reaction in the liquid phase. After the reaction, the reaction liquid was distilled under reduced pressure to obtain a yellow liquid, and then the liquid was poured into 100 mL of water, extracted 3 times with 150 mL of dichloroethane, the organic phase was collected, and the solvent was removed by distillation under reduced pressure. Obtained 20 g of light yellow liquid, yield: 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com