Stress resistant plant in which cell death suppressing gene is introduced and method for producing same
A technology of cell death and gene suppression, applied in the field of cultivating stress-resistant plants, which can solve the problems of unresearched resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: Preparation of plant expression vector
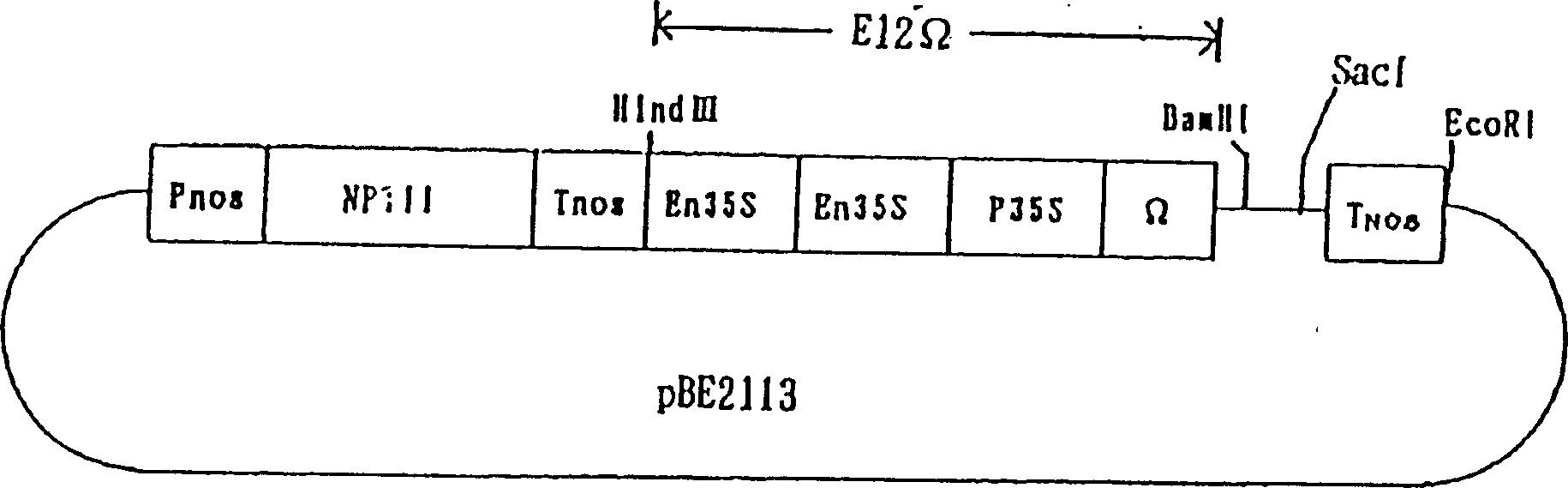
[0077] figure 1 The indicated bidirectional vector pBE2113 was used as starting material for plant expression vectors. As described in Japanese Laid-Open Publication No. 7-250685, bidirectional vector pBI121 (manufactured by Clontech) containing drug-resistant gene regions (Pnos, NPTII, and Tnos) and into which the E12Ω promoter region sequence was introduced was used as a starting material. Plant expression vector.
Embodiment 2
[0078] Example 2: Isolation of cell death suppressor genes from animals and construction of plant expression vectors
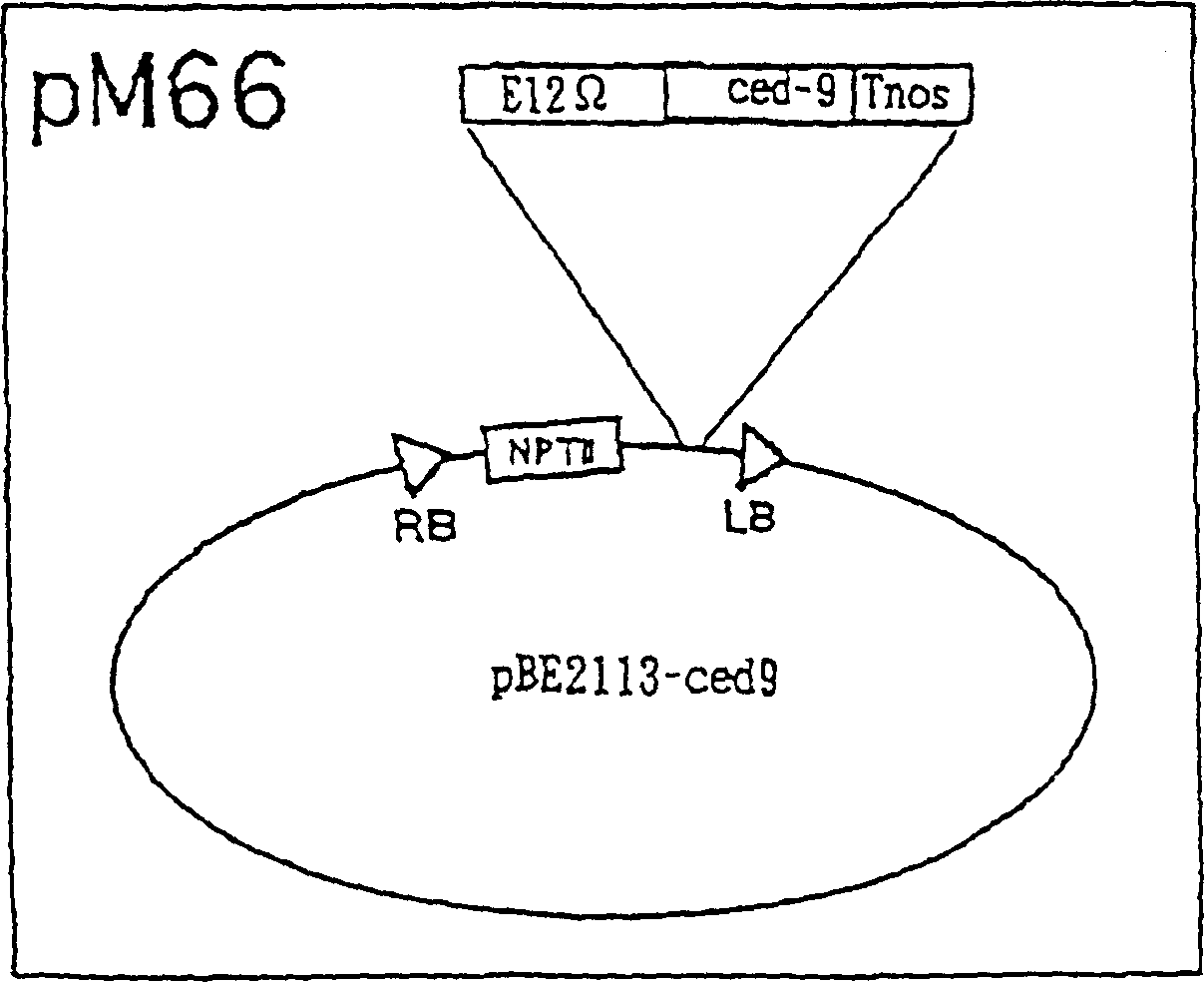
[0079] Total RNA was isolated from C. elegans using TRIsol (Life Technologies) and first-strand cDNA was synthesized from mRNA. Then, ced-9 full-length cDNA was synthesized by RT-PCR using 5'-TTGAATTCGAGATGACACGCTGCACGGCGG-3' (SEQ ID NO: 1) as a primer. Then, PCR was performed using Pfu polymerase (manufactured by Stratagene) while using SEQ ID NO: 1 as a 5' primer and 5'-GGGAATTCGTTACTTCAAGCTGAACATCAT-3' (SEQ ID NO: 2) as a 3' primer. The DNA was denatured at 94°C for 1.5 minutes, annealed at 55°C for 2.5 minutes and extended at 72°C for 2 minutes, and this cycle was repeated 25 times. The PCR product was cloned into the EcoRI site of pBluescript to obtain plasmid pM61.
[0080] On the other hand, using 5'-ATGTCTCAGAGCAACCGGGAGCTGGTGGTT-3' (SEQ ID. NO: 6) as the 5' primer, and 5'-TCATTTCCGACTGAAGAGTGAGCCCAGCAG-3' (SEQ ID NO: 7) as the 3' primer, the human c...
Embodiment 3
[0081] Example 3: Introduction of expression vectors into tobacco plants
[0082] Transformation of Agrobacterium tumefaciens
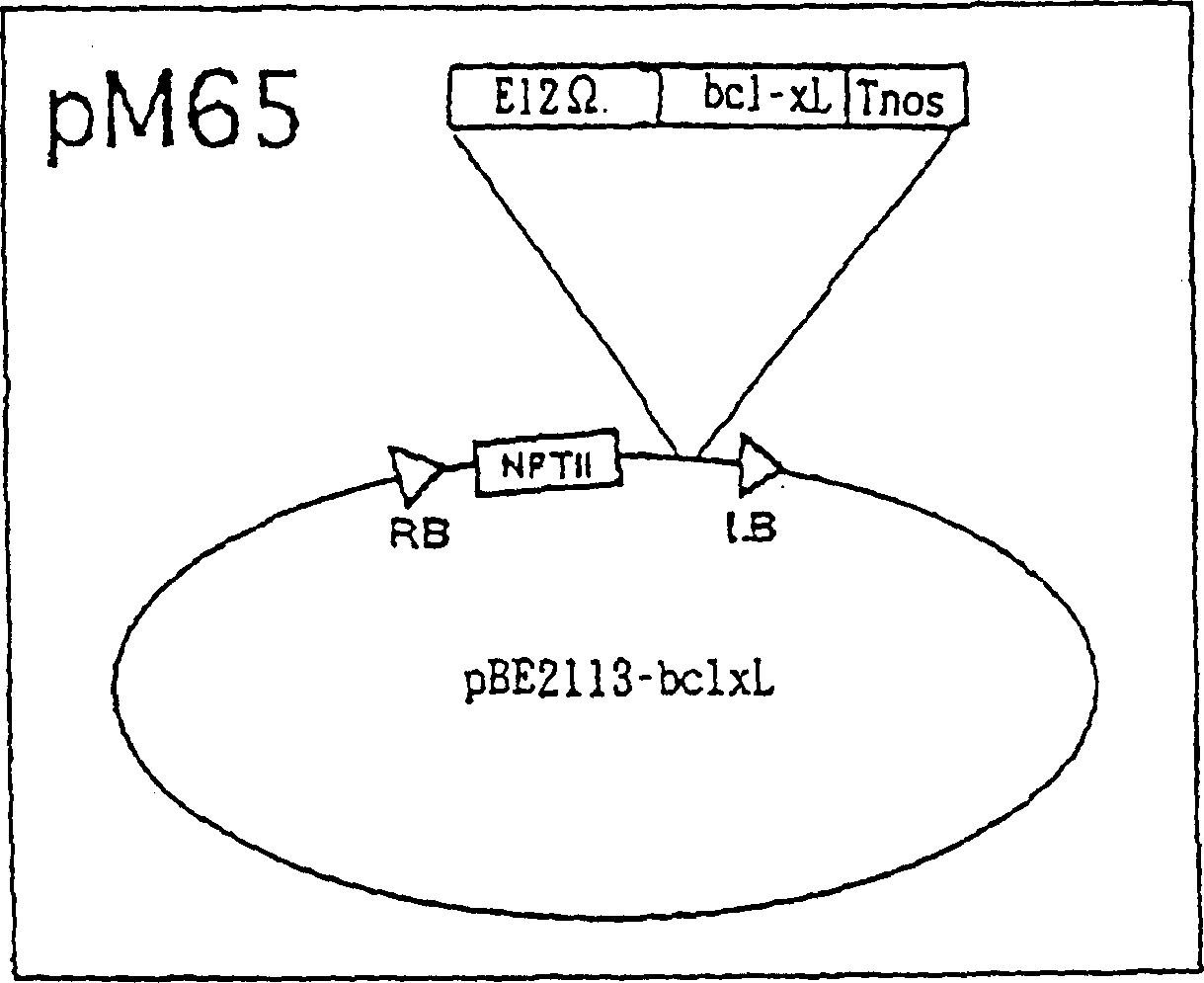
[0083] Agrobacterium tumefaciens were cultured at 28°C in a medium containing 250 μg / ml streptomycin and 50 μg / ml rifampicin. Cell suspension cultures were prepared, and the expression vector (pM65 or pM66) was introduced into the above-mentioned bacteria by electroporation according to the method described by Navel et al., Microbiology Communications, 67, 325, 1990.
[0084] In one example, a plasmid (pBI121:35S-GUS) containing the GUS (glucuronidase) gene linked to the CaMV-35S promoter was used, and in another example, a plasmid containing the gene fused to the CaMV 35S promoter was used. The plasmid (35S-POX) of the POX (superoxidase from rice) gene (Ito et al., Plant Cell Reports, 13:361-366, 1994) was transformed in the same manner to compare the transformation efficiency.
[0085] transformation of tobacco
[0086] Agrobacterium transformed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com