Biomarker for saliva test of 2019-nCoV and application of biomarker
A 2019-ncov and 2019ncov technology, applied in the field of molecular biology, can solve problems such as difficult constant standardization, false negative detection, and operators' susceptibility to infection, and achieve simple sampling, high sensitivity and specificity, and good reliability and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The purification of trace nucleic acid in the saliva of embodiment 1
[0094] 1. Sample source:
[0095] Shanghai Public Health Clinical Center
[0096] Collection method: before collection, the patient rinses mouth with water 3-4 times to remove oral mucus and food residue. After gargling, stand still for 3-5 minutes, secrete 1.5-2 ml of saliva under the tongue or from both cheeks, and spit it into a saliva collection tube with a cover. The collected samples should be placed in a refrigerator at 4 degrees Celsius immediately.
[0097] A total of 25 cases were collected.
[0098] 2. Use the following steps to purify trace amounts of nucleic acid in saliva:
[0099] 1) Rinse mouth with water 3-4 times to remove food residue and mucus in the mouth; stand still for 3 minutes.
[0100] 2) Spit 1.5-2 ml of saliva into a capped experimental tube treated with high temperature and high pressure.
[0101] 3) Transfer the sample to a 1.5 ml high temperature and high pressure...
Embodiment 2
[0112] Embodiment 2 purification result analysis
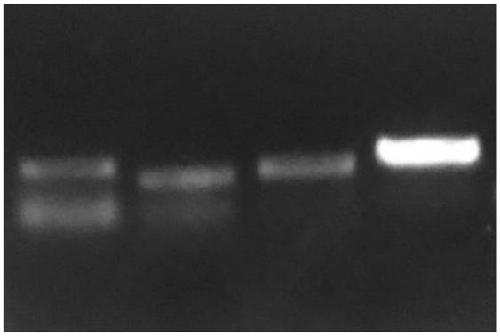
[0113] The purified saliva supernatant nucleic acid generates cDNA (Qiagen reverse transcription kit) after reverse transcription, and then uses the specific amplification primer of coronavirus gene S protein to the amplification of the nucleic acid sample PCR that obtains in embodiment 1 (PCR), the sequence of the specific amplification primer with coronavirus gene S protein is:
[0114] Upstream primer: (SEQ ID NO: 1): Downstream primer: (SEQ ID NO: 2):
[0115] Table 1 Amplification reaction system
[0116] dNTP (2.5μM) 160μL 10X EX buffer 2.0 μL specific primer 200μL template DNA 1.0 μL Teq DNA polymerase 10μL PCR buffer (10 times working concentration) 200μL
[0117] (2) The results of PCR were carried out by agarose gel electrophoresis, and the results were as follows: figure 1 As shown, the result proves that:
[0118] According to the method of the present inventio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com